Application of protein SFTPC as lung cancer diagnostic marker and kit
A kit and protein technology, applied in the field of medical diagnosis, can solve the problems of ineffective enrichment of exosomes from tissues or diseases, inability to reflect the specificity of exosomes, complex sources of exosomes, etc., and achieve great theoretical value and clinical application value, good clinical application prospects, and structurally complete effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
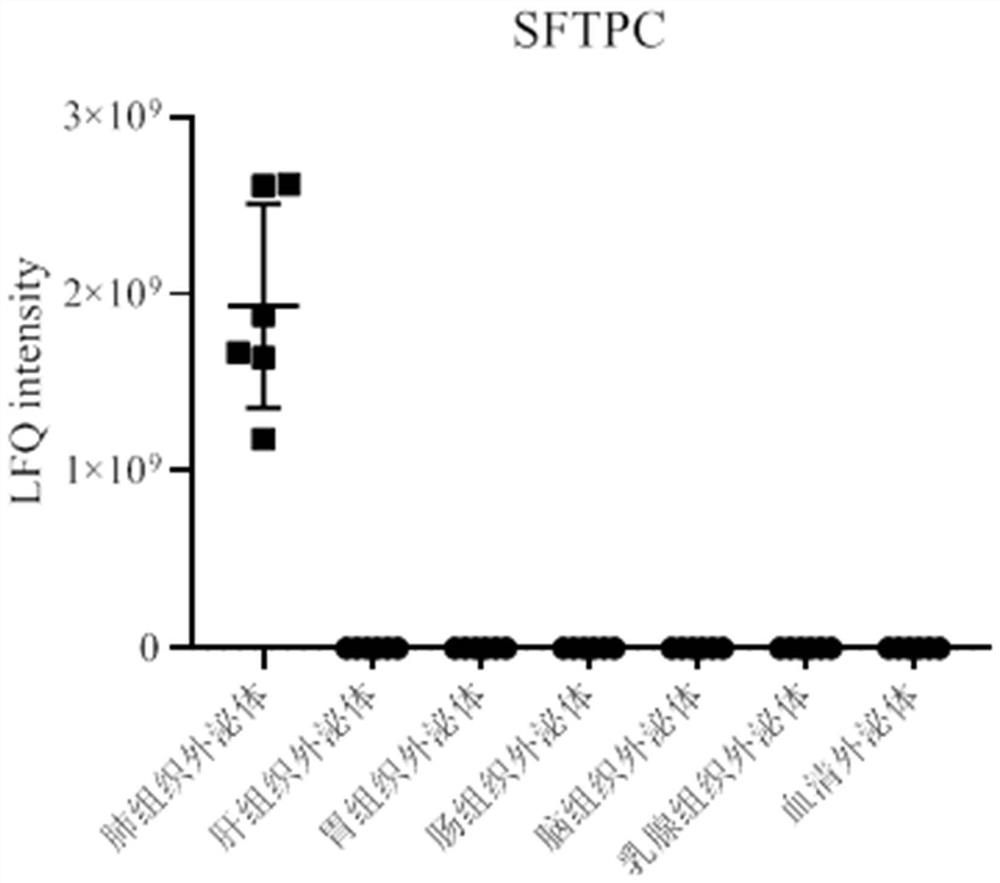
[0066] Example 1: Discovery of the lung tissue exosome surface marker SFTPC
[0067] Methods as below:
[0068] Proteomic analysis of exosomes from different types of tissue sources (lung tissue, liver tissue, gastric tissue, intestinal tissue, brain tissue, breast tissue) and serum exosomes was performed using mass spectrometry. see results figure 1 ,Specific steps are as follows:
[0069] 1. Preparation of tissue exosomes
[0070] (1) Rinse tissue samples with PBS to remove blood stains, remove fat tissue, connective tissue, necrotic tissue and other redundant tissues and the original storage medium;
[0071] (2) Transfer the tissue sample to a petri dish, add an appropriate amount of PBS, and cut the tissue sample with ophthalmic scissors;
[0072] (3) Add collagenase so that the concentration of the working solution is 1 mg / mL, place on a constant temperature shaker at 37°C, 90 rpm, and incubate for about 60 minutes until the tissue block has good light transmission an...
Embodiment 2
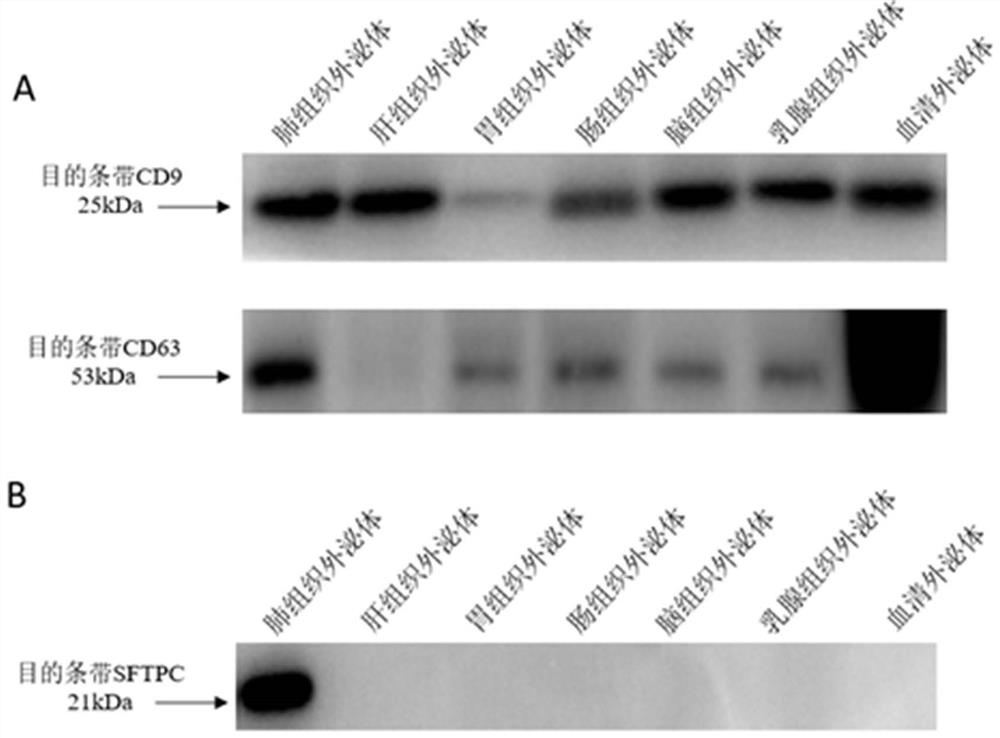
[0120] Example 2: Verification of the lung tissue exosome surface marker SFTPC
[0121] Methods as below:
[0122] Take the PBS resuspension of tissue exosomes and serum exosomes, add an equal volume of RIPA (strong) lysate for lysis and protein extraction, and detect exosome markers and SFTPC by Western blot. see results figure 2 ,Specific steps are as follows:
[0123] (1) Gel preparation: according to the molecular weight of the target protein, use a 1.5 mm glass plate and a 15-hole sample comb to prepare a 10% separating gel and 5% stacking gel;
[0124] (2) Sample loading
[0125] (3) Electrophoresis: conduct electrophoresis at a steady voltage of 80 v until the Loading Buffer (indicator) enters the separation gel, change to a constant voltage of 120 v and continue electrophoresis until the Loading Buffer (indicator) reaches the bottom of the gel, then the electrophoresis can be terminated;
[0126] (4) Membrane transfer: A PVDF membrane with a pore size of 0.22 um w...
Embodiment 3
[0134] Example 3: Specific verification of lung tissue exosome surface marker SFTPC
[0135] Methods as below:
[0136] Use the public database Human Protein Atlas (http: / / www.proteinatlas.org / ) and GTEx database (https: / / www.gtexportal.org / home / ) to query the expression of mass spectrometry screening index SFTPC in different tissues, query results It shows that SFTPC is highly specifically expressed only in lung tissue.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com