Method for synthesizing N-methyl carbamate
A technology of methyl carbamate and methanol, which is applied in the preparation of carbamic acid derivatives, chemical instruments and methods, and the preparation of organic compounds. It can solve the problems of environmental protection treatment of invalid catalysts, increase production costs, and affect product purity, etc. problems, achieve good commercial value, reduce the trouble of separation process, and improve the effect of production safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
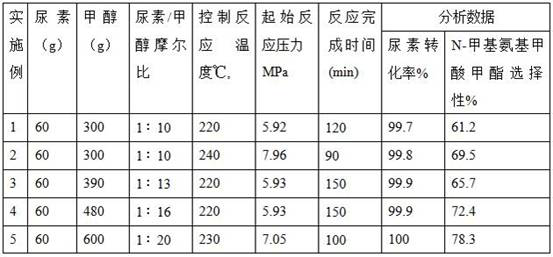
[0016] Add 60g (1mol) urea and 300g methanol to a 1L high-pressure reactor with an alcohol ammonia separator, seal the reactor, fill the reactor with nitrogen to 0.6MPa, discharge to 0.1MPa, replace 4 times continuously, and keep the last time 0.1 MPa nitrogen residual pressure.
[0017] After checking that the valves of the reaction kettle and the alcohol and ammonia separator are in the correct position, turn on the agitator (100-200 rpm), turn on the electric heating system, and raise the temperature of the material to 220°C at a heating rate of 3-4°C / min , the equilibrium pressure at this temperature is 5.92MPa. After maintaining the temperature for 15 minutes, methanol, ammonia, and carbon dioxide are separated by the alcohol ammonia separator, and the methanol is returned to the reactor. The ammonia and carbon dioxide generated by the reaction are continuously discharged from the reaction system and introduced into the absorption device.
[0018] When the temperature of...
Embodiment 2
[0021] Other processing conditions are with embodiment 1, and the raw material that adds and temperature of reaction are as follows:
[0022] Add 60 g of urea and 300 g of methanol, and the reaction temperature is 240° C. to obtain 61.9 g of the product.
Embodiment 3
[0024] Other processing conditions are with embodiment 1, and the raw material that adds and temperature of reaction are as follows:
[0025] Add 60 g of urea and 390 g of methanol, and the reaction temperature is 220° C. to obtain 58.5 g of the product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com