A kind of 3-hydroxybutyrylation modified protein drug and its preparation method and application
A protein and drug technology, applied in the field of medicine, can solve the problems of protein-limited medicine and commercial applications, antibody and receptor loss of antibody target cells, Fc fragment fragmentation, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0084] Example 1: Recombinant expression of antibody (antibody preparation refers to "Wu, Y., et al., A noncompeting pair of human neutralizing antibodies block COVID-19 virus binding to its receptor ACE2. Science, 2020. 368(6496): p. 1274-1278." Antibody B38 Preparation)
[0085] 1.1 Plasmid extraction
[0086] (1) Take 400mL of E. coli cultured overnight, centrifuge at 9000r / min for 10min, drain the supernatant as much as possible, and collect the bacteria;
[0087] (2) Resuspend the bacterial pellet with 25mL solution P1, and vortex until completely suspended;
[0088] (3) Add 25mL of solution P2, gently turn it up and down 4-7 times to fully lyse the bacteria, and place it at room temperature for 4 minutes;
[0089] (4) Add 25mL of solution P3, turn it up and down gently 4-7 times immediately, and mix well, at this time, a white flocculent precipitate will appear; let stand on ice for 3-5min, centrifuge at 13000r / min for 10min, and carefully take the supernatant to...
Embodiment 2
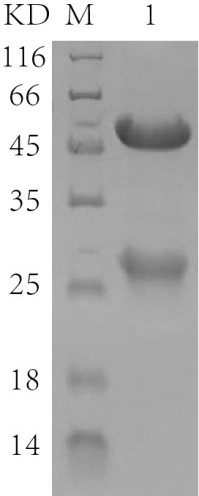
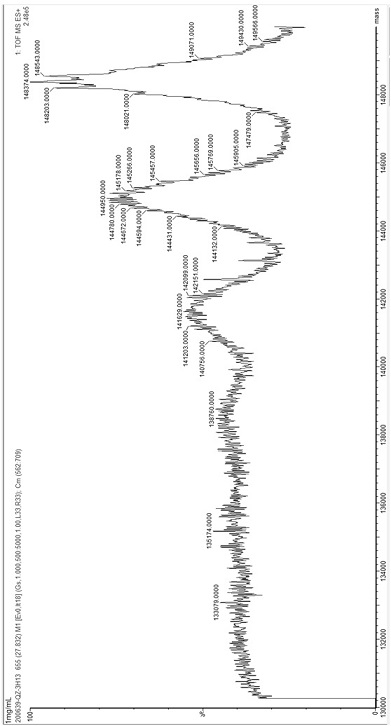
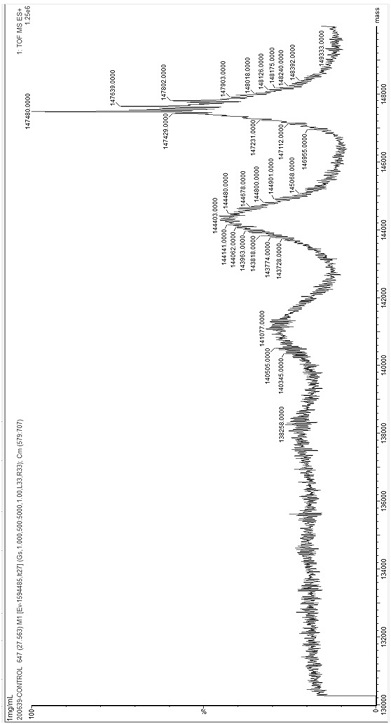
[0116] Example 2: Molecular weight detection after 3-hydroxybutyric acid modification of antibodies
[0117] Adjust the concentration of the antibody obtained in Example 1 to 1 mg / mL, add 1M 1-ethyl-(3-dimethylaminopropyl) carbodiimide hydrochloride and 1M 3-hydroxybutyric acid to a final concentration of 10 mM, React at room temperature for 2 hours, and then centrifuge 4 times with a 10kd ultrafiltration column to wash away unreacted compounds and perform mass spectrometry analysis.
[0118]The desalted antibody was made into a 0.2 mg / mL solution with 0.1% formic acid solution for flow injection analysis. Antibody protein samples for LC-MS analysis were directly prepared with ultrapure water, and 2 μL protein samples were injected directly with ultrapure water, and a micro syringe was used to draw protein samples, and the nanoACQUITY UPLC system was used to pass through the nanoACQUITY UPLC system at a flow rate of 1 μL / min for 30 minutes. Gradient elution separates the anal...
Embodiment 3
[0119] Example 3: Detection of modified sites after 3-hydroxybutyric acid modification of antibodies
[0120] The antibody obtained in Example 2 was detected by LC-MS / MS. Proteins were separated by SDS-PAGE, the gel band of interest was excised from the gel, reduced with 5 mM dithiothreitol, and alkylated with 11 mM iodoacetamide. Then, in-gel digestion was performed overnight at 37°C with sequencing-grade modified trypsin in 50 mM ammonium bicarbonate. Peptides were extracted twice with 0.1% trifluoroacetic acid in 50% acetonitrile in water for 30 minutes each. Tryptic peptides were redissolved in 20 μL 0.1% TFA and analyzed by LC-MS / MS. For LC-MS / MS analysis, peptides were separated by 85 min gradient elution at a flow rate of 0.30 μL / min using a Thermo-Dionex Ultimate 3000 HPLC system directly coupled to a Thermo Scientific QExactive mass spectrometer. The analytical column is a self-made fused silica capillary column (inner diameter 75 µm, length 150 mm, Upchurch) fille...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com