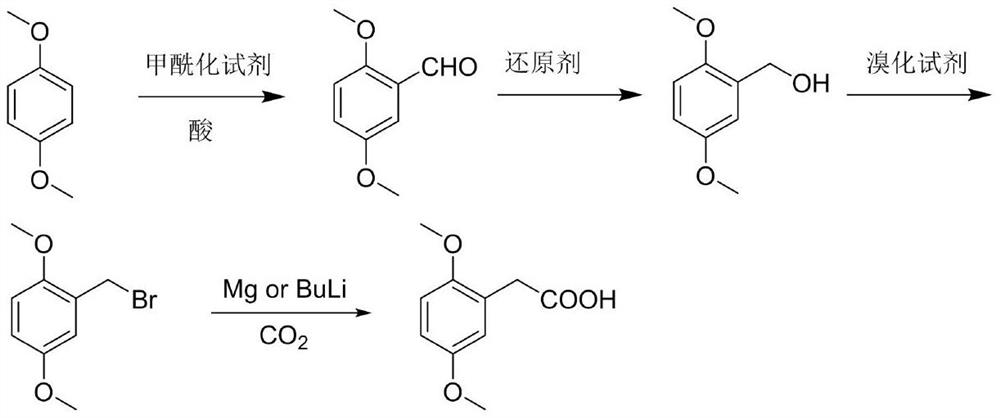
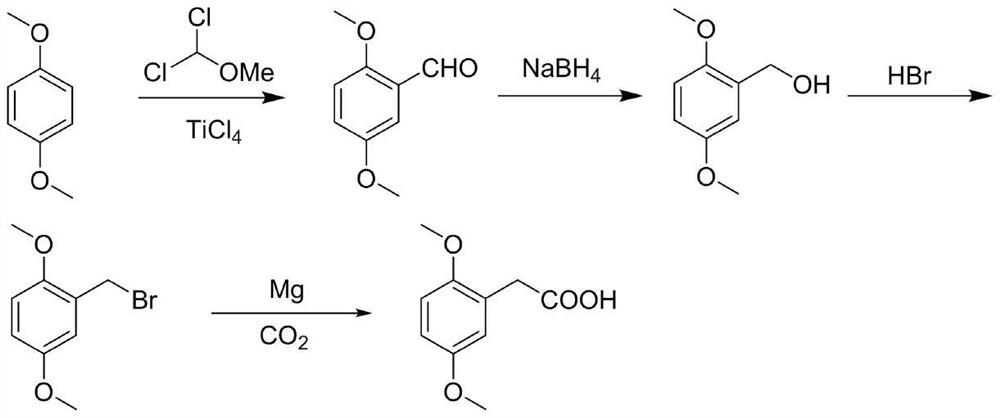
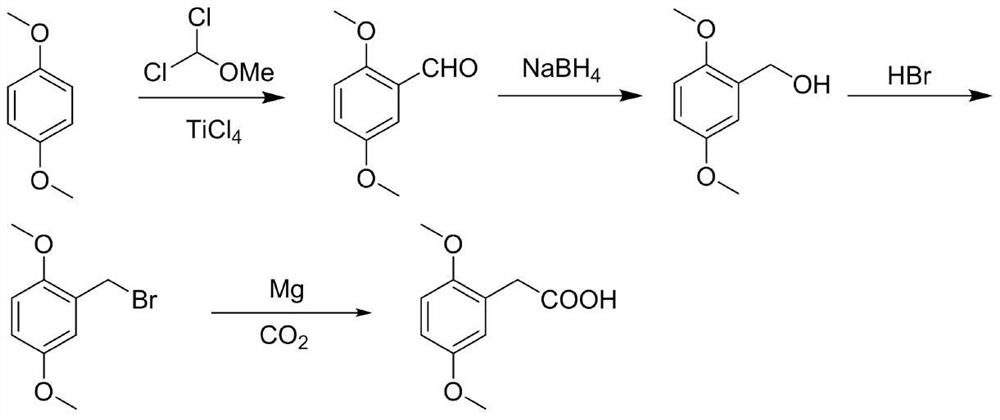
Preparation method of 2,5-dimethoxyphenylacetic acid
A technology of dimethoxybenzene and dimethoxybenzaldehyde, applied in the field of drug synthesis, can solve the problems of good safety, difficulty in meeting market demands and high cost, and achieve the effect of being beneficial to environmental protection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025]
[0026] A. Add 13.82g (0.1mol) of 1,4-dimethoxybenzene and 200mL of anhydrous dichloromethane to a 500mL three-necked round-bottomed flask. 0.12mol) of anhydrous dichloromethane solution 45mL, dropwise added 90mL of anhydrous dichloromethane solution dissolved in 1,1-dichloromethyl ether (16.09g, 0.14mol), added dropwise for 1.5h, and stirred at room temperature for 30min , add 300g ice cubes, 8mL concentrated hydrochloric acid to the reaction solution, stir for 1h, separate the liquids, extract the aqueous phase with dichloromethane twice, 100mL each time, combine the organic phases, wash the organic phase twice with water, 100mL each time, anhydrous Dry over sodium sulfate and concentrate under reduced pressure to obtain 16.57 g of oily product 2,5-dimethoxybenzaldehyde, yield 98%, HPLC 97.9%;
[0027] B. Add 10g (0.060mol) of 2,5-dimethoxybenzaldehyde and 80mL of methanol to a 500mL three-neck round bottom flask, cool down to 0°C, and add NaBH in batches 4 2.05g...
Embodiment 2
[0033]
[0034] A. Add 13.82g (0.1mol) of 1,4-dimethoxybenzene and 200mL of anhydrous dichloromethane into a 500mL three-necked round-bottomed flask. , 0.12mol) of anhydrous dichloromethane solution 45mL, dropwise added 90mL of anhydrous dichloromethane solution dissolved in 1,1-dichloromethyl ether (14.94g, 0.13mol), added dropwise for 1h, and stirred at room temperature for 30min , add 300g of ice cubes, 8mL of concentrated hydrochloric acid to the reaction solution, stir for 2h, separate the liquids, extract the aqueous phase with dichloromethane twice, 100mL each time, combine the organic phases, wash the organic phase with water twice, 100mL each time, anhydrous Dry over sodium sulfate and concentrate under reduced pressure to obtain 16.22 g of oily product 2,5-dimethoxybenzaldehyde, yield 97.6%, HPLC 97.9%;
[0035] B. Add 10g (0.060mol) of 2,5-dimethoxybenzaldehyde and 80mL of methanol to a 500mL three-neck round bottom flask, cool down to -10°C, and add NaBH in batc...
Embodiment 3
[0041]
[0042] A. Add 13.82g (0.1mol) of 1,4-dimethoxybenzene and 200mL of anhydrous dichloromethane into a 500mL three-necked round-bottomed flask. 0.12mol) of anhydrous dichloromethane solution 45mL, dropwise added 90mL of anhydrous dichloromethane solution dissolved in 1,1-dichloromethyl ether (17.2g, 0.15mol), added dropwise for 3h, and stirred at room temperature for 30min. Add 300g of ice cubes and 8mL of concentrated hydrochloric acid to the reaction solution, stir for 2h, separate the liquids, extract the aqueous phase with dichloromethane twice, 100mL each time, combine the organic phases, wash the organic phase with water twice, 100mL each time, anhydrous sulfuric acid Dry over sodium and concentrate under reduced pressure to obtain 16.31 g of oily product 2,5-dimethoxybenzaldehyde, yield 98.2%, HPLC 97.9%;
[0043] B. Add 10g (0.060mol) of 2,5-dimethoxybenzaldehyde and 80mL of methanol to a 500mL three-necked round-bottomed flask, cool down to 10°C, and add NaBH...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com