Copper coordination polymer as well as preparation method, crystal and application thereof
A polymer and copper coordination technology, applied in the chemical field, can solve the problems of antiferromagnetism, antibacterial, and antibacterial applications, and achieve good inhibitory effect, good stability, and good antibacterial performance.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
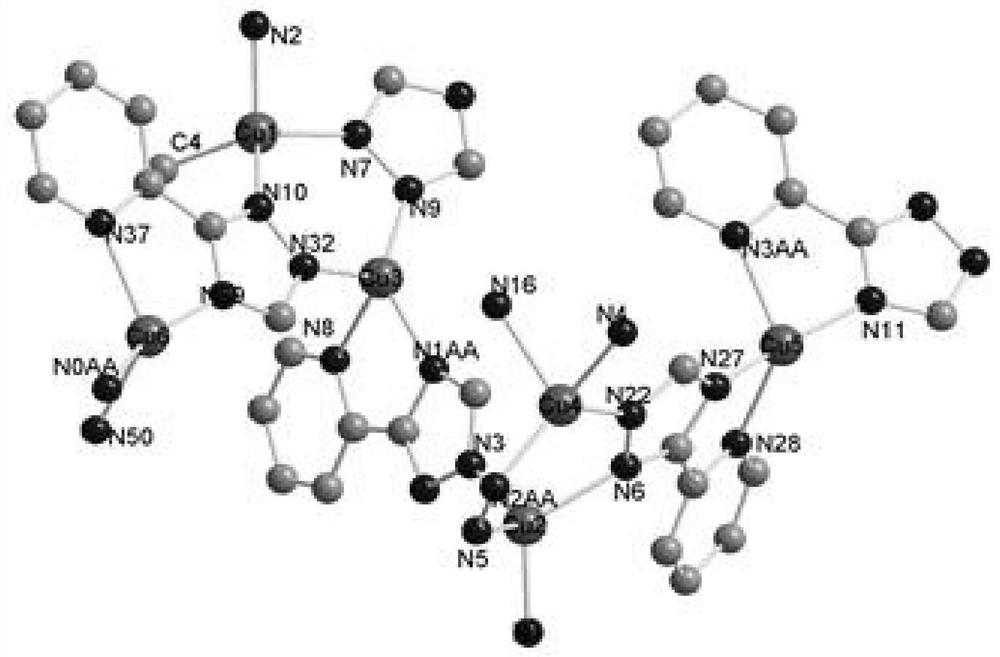
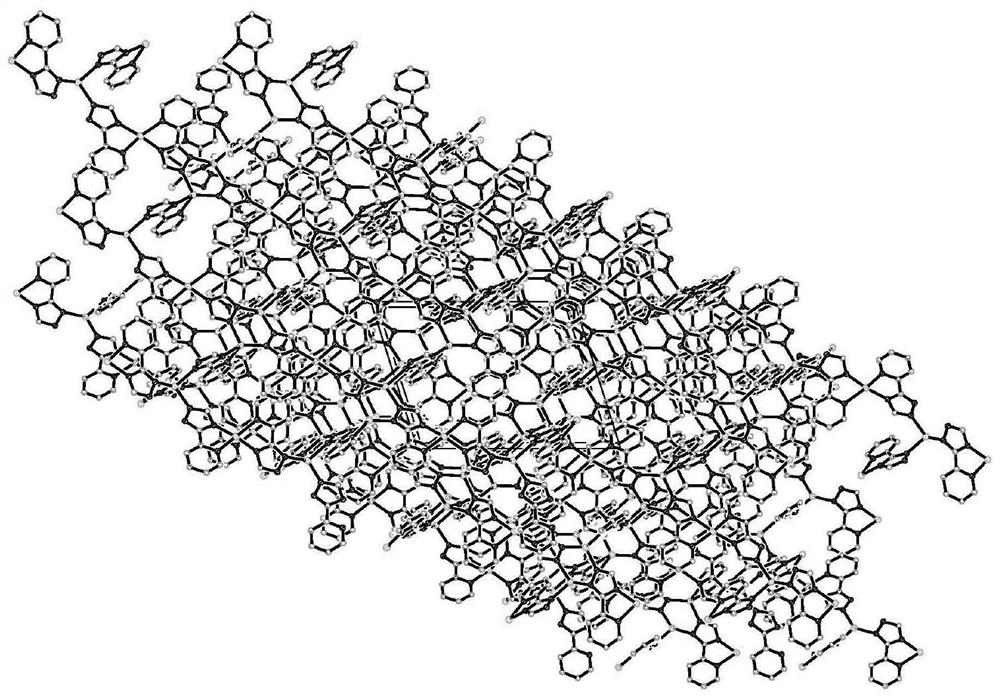
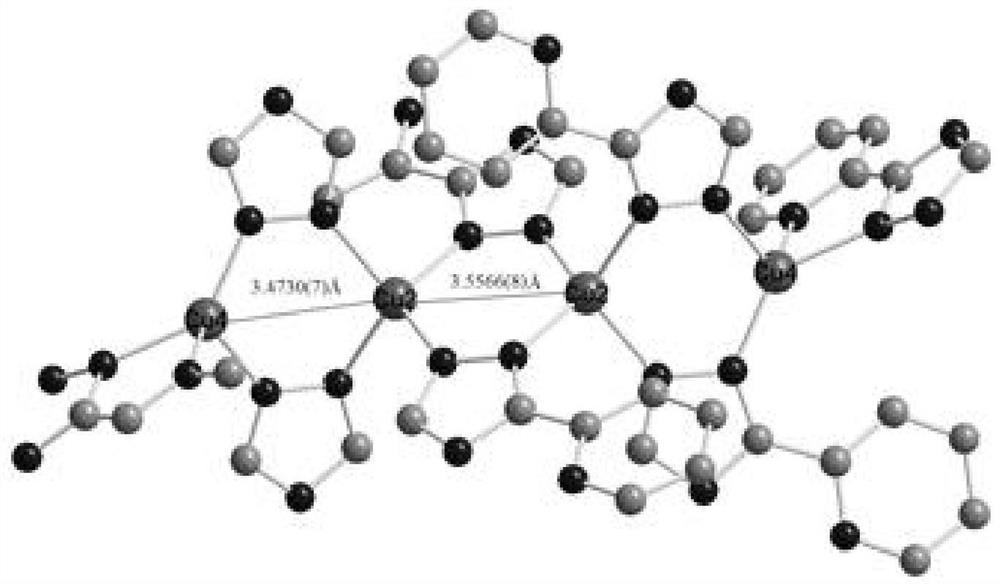
[0037] Embodiment 1 copper coordination polymer [Cu 6 (C 7 h 5 N 4 ) 6 ] n preparation of
[0038] Add 0.2mmol (48.0mg) 2,2′-biphenyldicarboxylic acid, 0.3mmol (about 28.7mg) cuprous bromide, 0.1mmol (14.6mg) 3–(2–pyridyl)–1,2, Add 4-triazole into a 30mL hydrothermal reaction kettle, a mixed solution of DMF and water (volume ratio 3:1), adjust the pH value to 6-7 with ammonia water, control the temperature at 140°C, and react for 72 hours. Speed drops to room temperature, obtains blue crystal, is described copper coordination polymer, productive rate 81.24%, fusing point: 278~280 ℃; Elemental analysis (C 84 h 60 Cu 12 N 48 ): Theoretical value (%): C, 40.22; H, 2.41; N, 26.81; Measured value (%): C, 40.14; H, 2.40; N, 26.89. The main absorption peaks of IR are: 3323(s), 1602.8(vs), 1483.2(vs), 1423.5(m), 1336.7(m), 1286.5(w), 1141.9(m), 1049.3(w), 1024.2(m) ,800.5(m),760.0(w),719.5(m),522.7(w).
Embodiment 2
[0039] Embodiment 2 copper coordination polymer [Cu 6 (C 7 h 5 N 4 ) 6 ] n preparation of
[0040] Add 0.3mmol (72.0mg) 2,2′-biphenyldicarboxylic acid, 0.2mmol (about 38.0mg) cuprous iodide, 0.1mmol (14.6mg) 3–(2–pyridyl)–1,2, Add 4-triazole into a 30mL hydrothermal reaction kettle, a mixed solution of acetonitrile and water (volume ratio 1:4), adjust the pH value to 6.5-7.5 with potassium hydroxide, control the temperature at 160°C, react for 48 hours, press Decrease to room temperature at a certain speed to obtain blue crystals, namely the copper coordination polymer, with a yield of 78.12%.
Embodiment 3
[0041] Embodiment 3 copper coordination polymer [Cu 6 (C 7 h 5 N 4 ) 6 ] n preparation of
[0042] Add 0.2mmol (48.0mg) 2,2′-biphenyldicarboxylic acid, 0.4mmol (about 39.6mg) cuprous chloride, 0.2mmol (29.2mg) 3-(2-pyridyl)-1,2, Add 4-triazole into a 30mL hydrothermal reaction kettle, a mixed solution of methanol and water (volume ratio 1:1), adjust the pH value to 5-6.5 with triethylamine, control the temperature at 150°C, react for 72 hours, press Decrease to room temperature at a certain speed to obtain blue crystals, namely the copper coordination polymer, with a yield of 80.32%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com