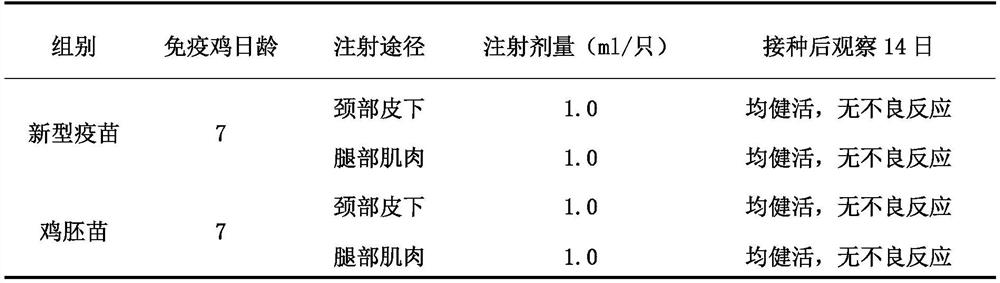
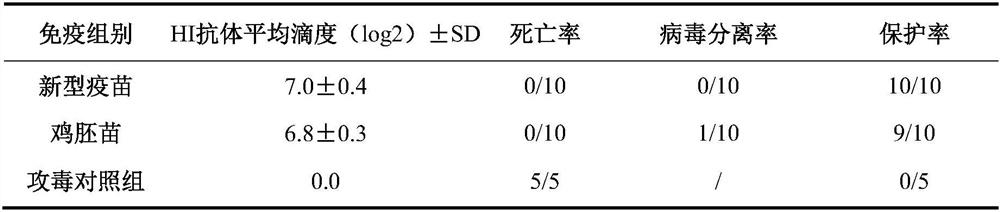
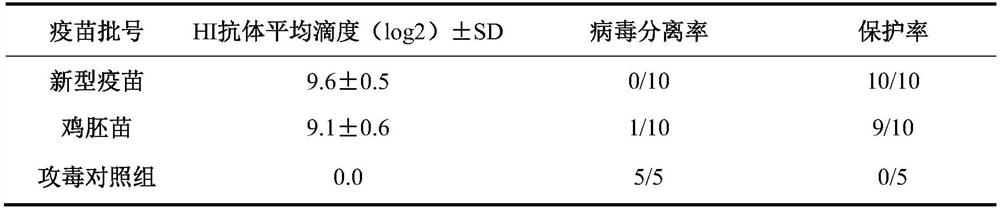
Triple inactivated vaccine for Newcastle disease, H9 subtype avian influenza and avian adenovirus
A technology for chicken Newcastle disease virus and avian influenza virus, which is applied in the field of poultry vaccines, can solve the problems of long production cycle, poor vaccine purity, and risk of spreading virus, and achieves the effect of high protection rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Embodiment 1 triple vaccine preparation of the present invention
[0042] 1 NDV cytotoxic preparation
[0043] AGE1 cells (duck embryonic retinal suspension cells) were gradually enlarged and passaged to a 7L bioreactor, the volume of the culture medium was 4-5L, the pH value was set to 7.15, the dissolved oxygen value was set to 60%, and the rotation speed was set to 150r / min, cultured at 37°C. When the cell density is 8.0×10 6 When the NDV aSG10 strain virus is inoculated at 0.0001 MOI, DMEM medium is added to dilute the DMEM medium concentration to 15% of the original concentration; and trypsin solution with a final concentration of 5 μg / ml is added daily. The same dose of trypsin solution was used once, the culture temperature was adjusted to 35°C, cultured for 72 hours, and samples were taken to detect the HA potency. When the HA potency was not lower than 8log2, the virus liquid was harvested. Freeze and thaw 3 times and set aside.
[0044] 2 H9 subtype avian...
Embodiment 2
[0060] Embodiment 2 triple vaccine preparation of the present invention
[0061] 1 NDV cytotoxic preparation
[0062] AGE1 cells (duck embryonic retinal suspension cells) were gradually enlarged and passaged to a 7L bioreactor, the volume of the culture medium was 4-5L, the pH value was set to 7.15, the dissolved oxygen value was set to 60%, and the rotation speed was set to 150r / min, cultured at 37°C. When the cell density is 8.0×10 6 When the NDV aSG10 strain virus is inoculated at 0.0001 MOI, DMEM medium is added to dilute the DMEM medium concentration to 10% of the original concentration; and trypsin solution with a final concentration of 3 μg / ml is added daily. The same dose of trypsin solution was used once, the culture temperature was adjusted to 36°C, cultured for 72 hours, and samples were taken to detect the HA potency. When the HA potency was not lower than 8log2, the virus liquid was harvested. Freeze and thaw 3 times and set aside.
[0063] 2 H9 subtype avian...
Embodiment 3
[0079] Embodiment 3 triple vaccine preparation of the present invention
[0080] 1 NDV cytotoxic preparation
[0081] AGE1 cells (duck embryonic retinal suspension cells) were gradually enlarged and passaged to a 7L bioreactor, the volume of the culture medium was 4-5L, the pH value was set to 7.15, the dissolved oxygen value was set to 60%, and the rotation speed was set to 150r / min, cultured at 37°C. When the cell density is 8.0×10 6 When the NDV aSG10 strain virus is inoculated at an MOI of 0.0001, DMEM medium is added to dilute the DMEM medium concentration to 20% of the original concentration; and trypsin solution with a final concentration of 7 μg / ml is added daily. The same dose of trypsin solution was used once, the culture temperature was adjusted to 37°C, cultured for 72 hours, and samples were taken to detect the HA potency. When the HA potency was not lower than 8log2, the virus liquid was harvested. Freeze and thaw 3 times and set aside.
[0082] 2H9 Subtype ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com