Preparation method of tofacitinib intermediate cis-1-benzyl-N, 4-dimethylpiperidine-3-amine dihydrochloride
A technology of dimethylpiperidine and amine dihydrochloride, which is applied to the intermediate cis-1-benzyl-N of tofacitinib, can solve the problems such as difficulty in obtaining raw materials, complex reaction system, pollution, etc. Low equipment requirements, process safety and environmental protection, and the effect of improving reaction yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
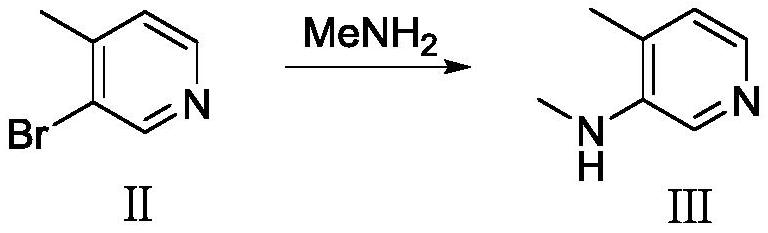
[0050] Preparation of compound Ⅲ
[0051]Add compound II (100g, 0.58mol) and 40% methylamine aqueous solution (350g) into the autoclave, add a catalytic amount of copper powder under stirring, slowly raise the temperature to 100-110°C, stir and react for 24h; Dichloromethane (500 mL) was extracted three times, dried and concentrated to obtain compound III (66 g, yield 93%).
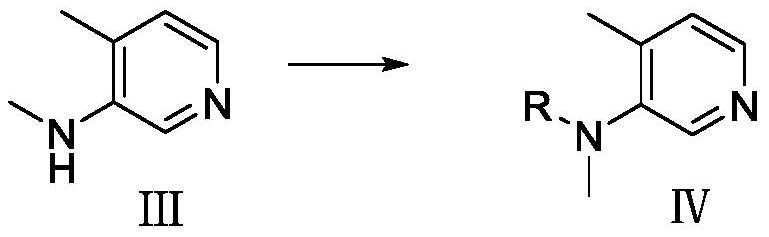
[0052] Preparation of compound Ⅳ
[0053] Add compound III (60g, 0.49mol) and dichloromethane (480mL) to the reaction flask, add N,N-dimethylaminopyridine (73g, 0.60mol), then add di-tert-butyl dicarbonate (123g, 0.56 mol); the temperature was raised to 50-55° C., stirred for 20 h, cooled to room temperature, and the organic phase was separated by water, dried and concentrated to obtain compound IV (95 g, yield 87%).
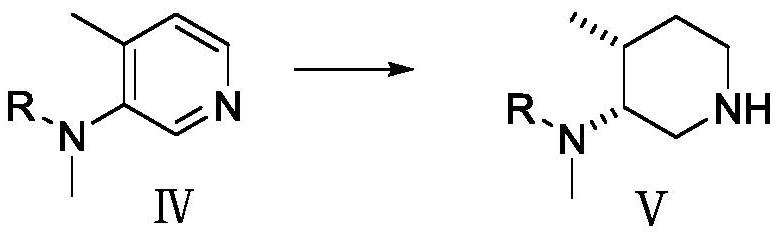
[0054] Preparation of Compound V
[0055] Add compound IV (95g, 0.43mol) to the autoclave, add glacial acetic acid (950mL), add 5% rhodium carbon (48g) after nitrogen replacement three t...
Embodiment 2
[0061] Preparation of compound Ⅲ
[0062] Compound II (100 g, 0.58 mol) and water (350 g) were added to the autoclave, followed by methylamine hydrochloride (196 g, 2.90 mol) and sodium hydroxide (120 g, 3.0 mol). A catalytic amount of copper powder is added with stirring. Slowly raise the temperature to 120-130°C, and stir the reaction for 24h. Cool down to room temperature, extract three times with dichloromethane (500 mL), dry and concentrate to obtain compound III (61.6 g, yield 87%).
[0063] Preparation of compound Ⅳ
[0064] Compound III (60g, 0.49mol) and tetrahydrofuran (480mL) were added to the reaction flask, sodium hexamethyldisilazide (600mL, 0.60mol, 1.0M) was added, and di-tert-butyl dicarbonate (123g, 0.56 mol); heated to 50-55 ° C, stirred for 24 hours, concentrated under reduced pressure to recover tetrahydrofuran, added dichloromethane and water to the residue, separated the organic phase, dried, and concentrated to obtain compound IV (98g, yield 90%) . ...
Embodiment 3
[0072] Preparation of compound Ⅲ
[0073] Add compound II (100g, 0.58mol) and 40% methylamine aqueous solution (300g) into the autoclave, and add a catalytic amount of copper powder under stirring; slowly raise the temperature to 110-120°C, and stir for 24h. Cool down to room temperature, extract three times with dichloromethane (500 mL), dry and concentrate to obtain compound III (65 g, yield 92%).
[0074] Preparation of compound Ⅳ
[0075] Add compound III (60g, 0.49mol) and dichloromethane (480mL) to the reaction flask, add triethylamine (60.7g, 0.60mol) and catalytic amount of N,N-dimethylaminopyridine in turn, then add ethyl Anhydride (57.2 g, 0.56 mol). The reaction was stirred at room temperature for 4h. Water was added to quench the reaction, and the organic phase was separated, dried and concentrated to obtain compound IV (80.5 g, yield 100%).
[0076] Preparation of Compound V
[0077] Compound IV (80 g, 0.49 mol) was added to the autoclave, methanol (800 mL) w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com