A kind of terbinafine hydrochloride effervescent tablet and using method thereof
A technology of soaking terbinafine hydrochloride and nafine, which is applied in the field of medicine, can solve the problems of long treatment time, adverse reactions, and reduced drug efficacy, and achieve the effects of wide range of applicable objects, prevention of drug loss, and short treatment time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
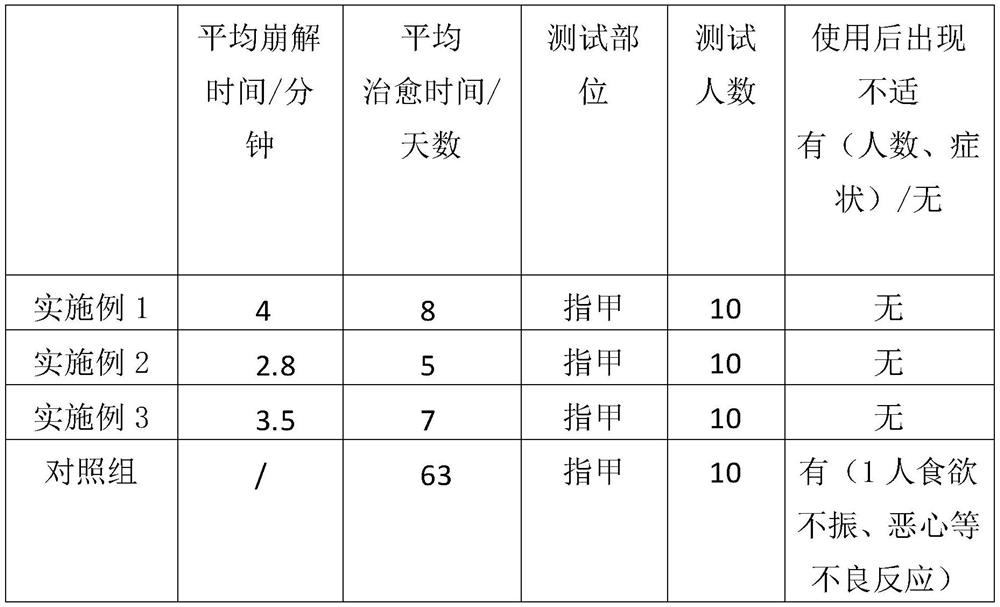
Embodiment 1
[0024] The invention provides a terbinafine hydrochloride effervescent tablet, wherein the main materials used include by weight: 120 parts of terbinafine hydrochloride, 160 parts of citric acid, 160 parts of sodium bicarbonate, sodium starch glycolate 20 parts and 15 parts of low-substituted hydroxypropyl cellulose, the auxiliary materials include by weight: 285 parts of lactose, 20 parts of polysorbate 80, 35 parts of povidone K30, 70 parts of anhydrous ethanol and 8 parts of magnesium stearate ;
[0025] Also includes the preparation method of terbinafine hydrochloride effervescent tablet, the specific operation steps are:
[0026] Step 1: raw material pretreatment, terbinafine hydrochloride, citric acid, sodium bicarbonate, sodium carboxymethyl starch, low-substituted hydroxypropyl cellulose and lactose are respectively dried, and then passed through a 100-mesh sieve respectively, according to the recipe quantity Weigh the spare;
[0027] Step 2: prepare a mixed solution...
Embodiment 2
[0037] The invention provides a terbinafine hydrochloride effervescent tablet, wherein the main materials used include by weight: 141 parts of terbinafine hydrochloride, 180 parts of citric acid, 180 parts of sodium bicarbonate, sodium starch glycolate 27.5 parts and 18.8 parts of low-substituted hydroxypropyl cellulose, the auxiliary materials include by weight: 305 parts of lactose, 25 parts of polysorbate 80, 47 parts of povidone K30, 81 parts of absolute ethanol and 10 parts of magnesium stearate ;
[0038]Also includes the preparation method of terbinafine hydrochloride effervescent tablet, the specific operation steps are:
[0039] Step 1: pretreatment of raw materials, terbinafine hydrochloride, citric acid, sodium bicarbonate, sodium starch glycolate, low-substituted hydroxypropyl cellulose and lactose were respectively dried, and then passed through a 100-mesh sieve respectively, according to the recipe quantity Weigh the spare;
[0040] Step 2: prepare a mixed solu...
Embodiment 3
[0050] The invention provides a terbinafine hydrochloride effervescent tablet, wherein the main materials used include by weight: 150 parts of terbinafine hydrochloride, 190 parts of citric acid, 190 parts of sodium bicarbonate, sodium starch glycolate 35 parts and 20 parts of low-substituted hydroxypropyl cellulose, the auxiliary materials include by weight: 315 parts of lactose, 30 parts of polysorbate 80, 55 parts of povidone K30, 90 parts of anhydrous ethanol and 12 parts of magnesium stearate ;
[0051] Also includes the preparation method of terbinafine hydrochloride effervescent tablet, the specific operation steps are:
[0052] Step 1: pretreatment of raw materials, terbinafine hydrochloride, citric acid, sodium bicarbonate, sodium starch glycolate, low-substituted hydroxypropyl cellulose and lactose were respectively dried, and then passed through a 100-mesh sieve respectively, according to the recipe quantity Weigh the spare;
[0053] Step 2: prepare a mixed soluti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com