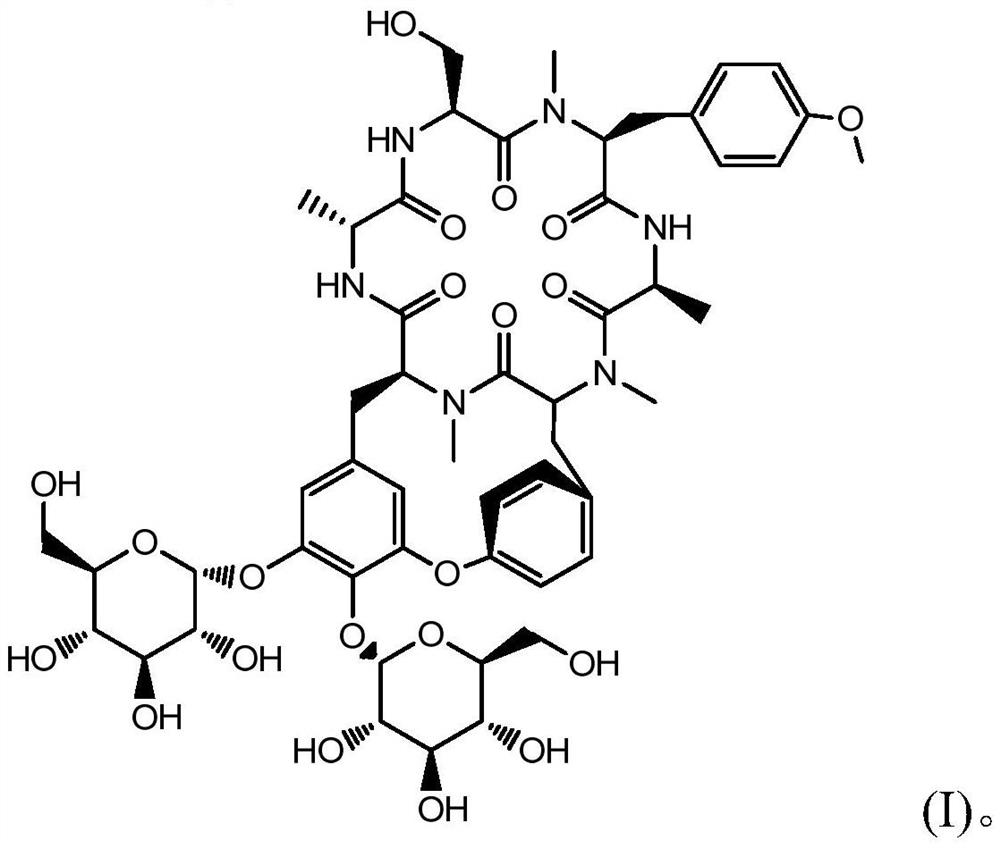
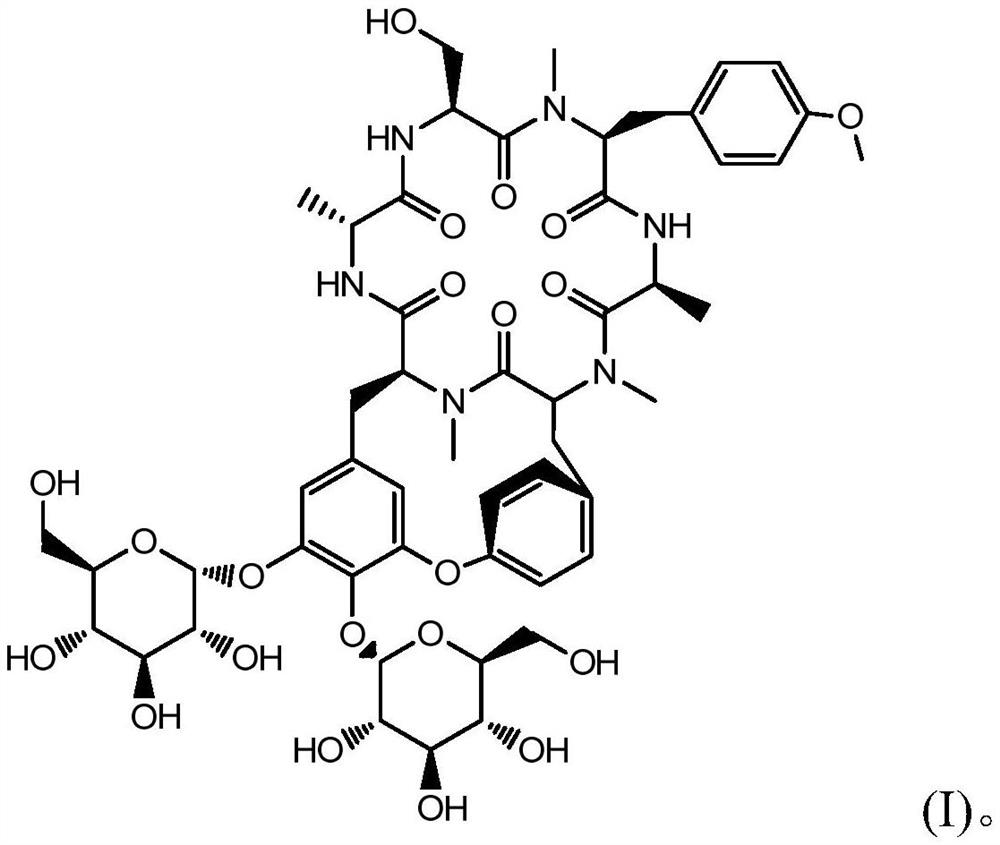
Radix rubiae cyclic peptide compound and preparation method thereof
A kind of compound, technology of Rubia genus, applied in the field of Rubia cyclopeptide compound and preparation thereof
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Embodiment 1: the preparation of target compound
[0031] 1) Take the dry stems and leaves (15.0kg) of Rubia hangii (Rubia hangii), grind them, and use 80v / v% methanol to reflux extract 3 times (60L×3 times), the time is 3 times each time hour, the extract was concentrated under reduced pressure to obtain an extract;
[0032] 2) Suspend the obtained extract by adding 15 L of methanol / water (the volume ratio of methanol to water is 50:50), and then extract with petroleum ether for 3 times, and the amount of solvent used for each extraction is 15 L. After extraction, take the methanol / water phase (lower phase), filter, remove the organic solvent under reduced pressure, add 9L of water and 3L of methanol, and put it on the XAD-16 macroporous resin column After loading, wash the column with water first, then elute with 30v / v% methanol, and finally elute with 100v / v% methanol. Collect the fraction eluted with 100v / v% methanol and concentrate under reduced pressure to obtai...
Embodiment 2
[0046] Embodiment 2: the preparation of target compound
[0047] Repeat Example 1, the difference is,
[0048] In step 1), the extraction medium is changed to 70v / v% ethanol; before the extract is extracted with petroleum ether, the extract is suspended in 15L of ethanol / water (the volume ratio of ethanol and water is 50:50).
[0049] In step 2), the model of the macroporous resin is changed to X-5. After loading the column, wash the column with water, then elute with 20v / v% ethanol, and finally elute with 100v / v% ethanol. Collect 100v / v% ethanol eluted fractions.
[0050] In step 3.1), measure 600 mL of n-hexane, 900 mL of ethyl acetate, 600 mL of methanol, and 900 mL of pure water to prepare a mixed solution with a volume ratio of 2:3:2:3. The layers were completely clarified, and the upper and lower layers were separated to obtain the upper phase solution and the lower phase solution, which were ultrasonically degassed for 10 min, respectively, and set aside.
[0051] Th...
Embodiment 3
[0053] Embodiment 3: the preparation of target compound
[0054] Repeat Example 1, the difference is,
[0055] In step 1), the extraction solvent is changed to water.
[0056] In step 2), the model of the macroporous resin was changed to DA101. After loading the column, the column was first washed with water, then eluted with 50v / v% methanol, and finally eluted with 90v / v% methanol. Fractions eluted with 90 v / v% methanol were collected.
[0057] The finally obtained fractions 18-19 are the molecular weight [M-H] - Compound 1095, detected by HPLC, has a content of 94.6%, is a white solid with a total weight of 45.3 mg, and is confirmed to be Yayehuanin A by 1HNMR and 13CNMR. Fraction 21 is molecular weight [M-H] - The compound of 1111 was detected by HPLC, and its content was 92.3%. It was a white powder with a total weight of 3.3 mg.
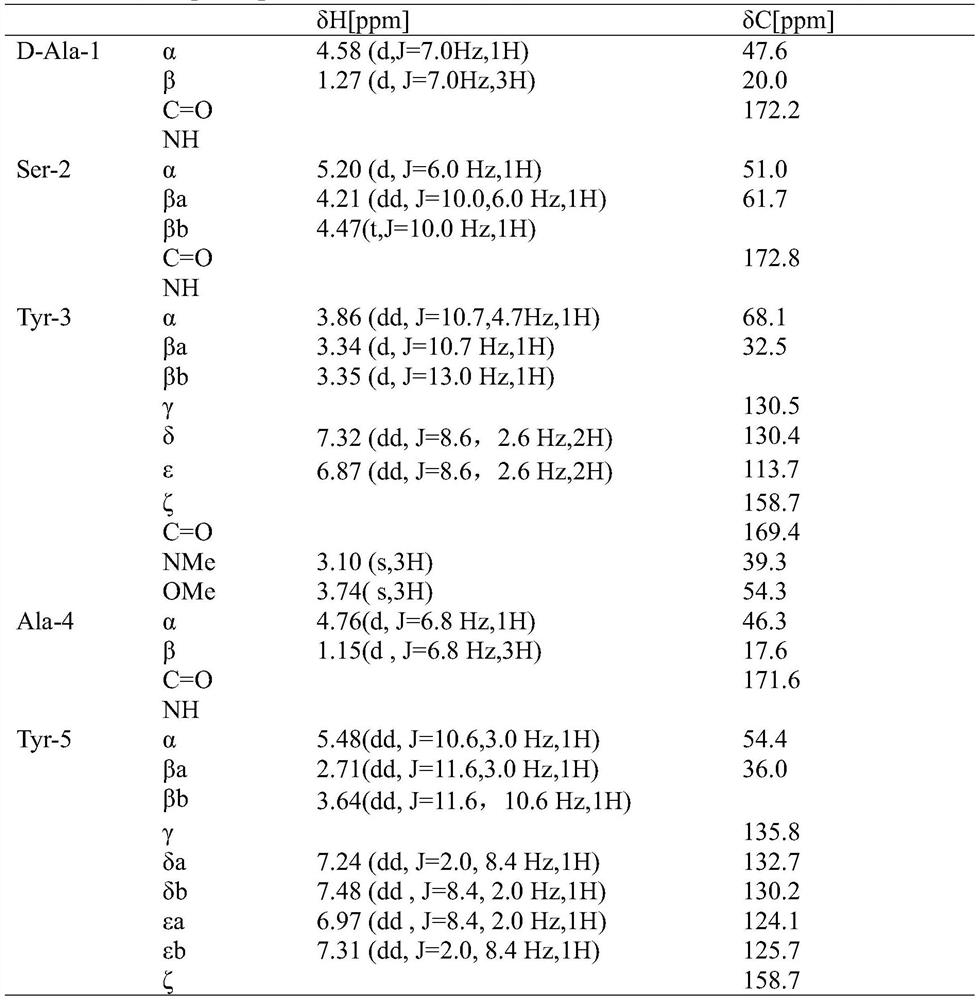
[0058] Molecular weight [M-H] obtained to the present embodiment - The white powder of 1111 was characterized by ultraviolet, hydrogen nu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com