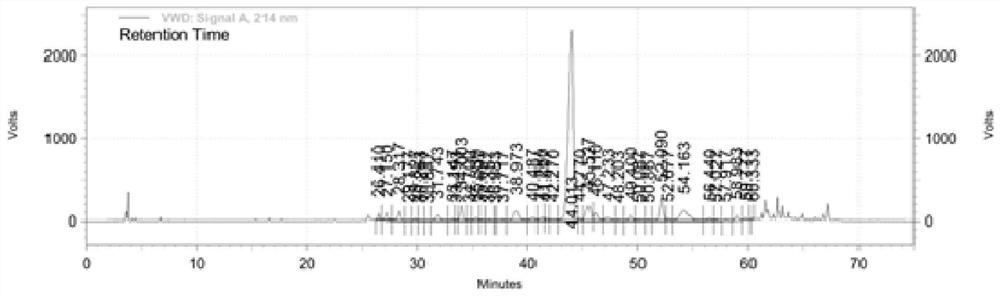
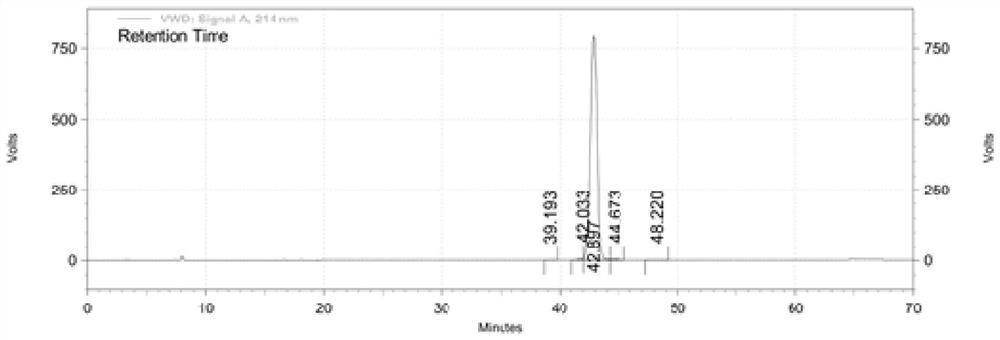
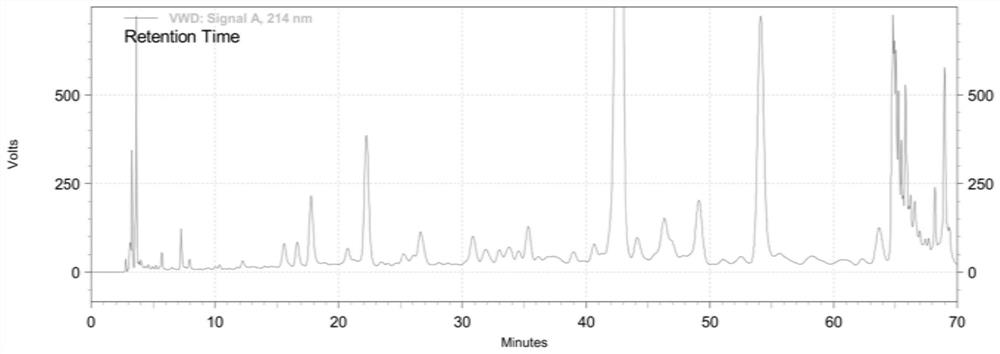
Synthetic method of semeglutide
A technology for solid-phase synthesis and resin synthesis, which is applied in the field of synthesis and purification of semaglutide, and can solve the problems of product yield, fragment condensed fragment solubility and condensation difficulty, and hydrophobic coupling of side chain octadecanedioic acid. It can avoid the formation of β-sheet secondary structure, improve the coupling effect, and reduce the production cost.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0040] The invention discloses a preparation method of semaglutide, and those skilled in the art can learn from the content of this article and appropriately improve the process parameters to realize it. In particular, it should be pointed out that all similar replacements and modifications are obvious to those skilled in the art, and they are all considered to be included in the present invention. The method and application of the present invention have been described through preferred embodiments, and the relevant personnel can obviously make changes or appropriate changes and combinations to the method and application described herein without departing from the content, spirit and scope of the present invention to realize and Apply the technology of the present invention.
[0041] The specific meanings of the abbreviations used in the specification and claims are as follows:
[0042]
[0043]
[0044] The raw materials, auxiliary materials and reagents used in the po...
Embodiment 1
[0046] The preparation of embodiment 1 Fmoc-Gly-Wang resin
[0047] Weigh 100 grams of Wang resin with a substitution degree of 0.8 mmol / g in a solid-phase reaction column, add DMF, and swell with nitrogen bubbles for 60 minutes; weigh 29.8 grams (100 mmol) of Fmoc-Gly-OH, and 16.2 grams (120 mmol) of HOBt , DMAP 1.2g (10mmol), dissolved in DMF, added 20.3mL DIC (120mmol) in ice-water bath at 0°C, activated for 5 minutes, added to the reaction column, reacted for 1.5 hours, added 70mL acetic anhydride and 60mL pyridine, mixed and blocked for 24 hours , washed three times with DCM, dried the resin after shrinking with methanol to obtain a total of 112 grams of Fmoc-Gly-Wang Resin, and the detected substitution degree was 0.31mmol / g.
Embodiment 2
[0048] The preparation of embodiment 2 Fmoc-Gly-Wang resin
[0049] Weigh 100 grams of Wang resin with a substitution degree of 1.0 mmol / g in a solid-phase reaction column, add DMF, and swell with nitrogen bubbles for 60 minutes; weigh 20.8 grams (70 mmol) of Fmoc-Gly-OH, and 11.3 grams (84 mmol) of HOBt , DMAP 0.8g (7mmol), dissolved in DMF, 28.5mL DIC (168mmol) was added in an ice-water bath at 0°C, activated for 5 minutes, added to the reaction column, after 2 hours of reaction, added 70mL of acetic anhydride and 60mL of pyridine, mixed and blocked for 24 hours , washed three times with DCM, dried the resin after shrinking with methanol, and obtained a total of 108 grams of Fmoc-Gly-Wang Resin, and the detection substitution degree was 0.23mmol / g.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com