Preparation method of lithium ferrite
A technology of lithium ferrite and iron source, applied in chemical instruments and methods, electrode manufacturing, iron compounds, etc., can solve the problem of high impurity content in products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0035] The embodiment of the present invention provides a preparation method of lithium ferrite, which comprises the following steps:
[0036] S1. Mixing iron source, lithium source and organic acid in a solvent to obtain a mixed solution;
[0037] S2, the mixed solution is heated to obtain a precursor solution;
[0038] S3. The precursor solution is dried and sintered to obtain lithium ferrite.
[0039] In the preparation method of lithium ferrite provided in the embodiment of the present invention, on the one hand, the iron source, lithium source and organic acid are mixed in an aqueous solvent by using a solvent method to obtain iron hydroxide colloid with a smaller particle size. In this process, since the lithium source is an alkaline compound, it is easy to make the ferric hydroxide colloid in the reaction system become ferric hydroxide precipitate, so an organic acid needs to be added to reduce the generation of ferric hydroxide precipitate. At the same time, the organi...
Embodiment 1
[0056] This embodiment provides a kind of preparation method of lithium ferrite, the steps are as follows:
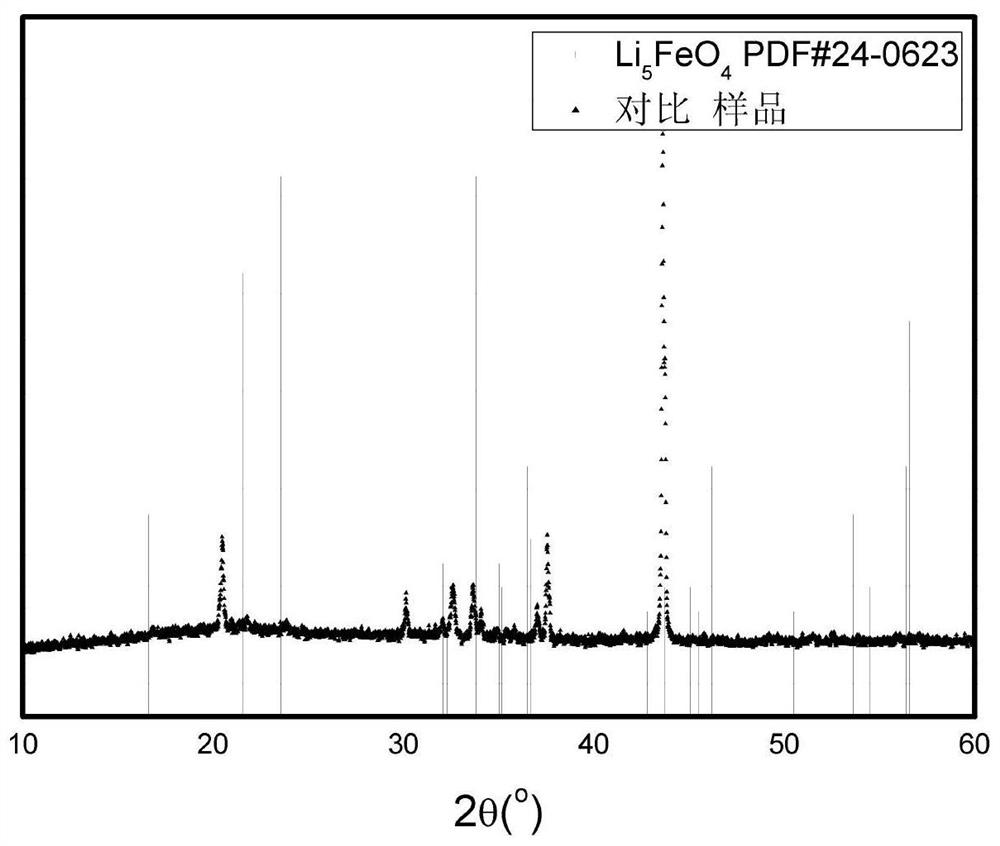
[0057] Fe(NO 3 ) 3 9H 2 O 0.10mol was dissolved in a mixed solvent of 100mL distilled water and 20mL ethanol, and stirred in an ice-water bath. Take 3mol / L LiCH 3 COO and 1mol / L Li 2 CO 3 The solution was slowly dropped into the above solution, so that the LiCH 3 The amount of substance added by COO is 0.20mol; Li 2 CO 3The amount of the substance added is 0.20 mol, and oxalic acid is added during the titration process to ensure that the pH is less than or equal to 3.7. Then put it into a reaction kettle, and carry out a solvothermal reaction at 150° C. After the reaction is completed, the solution is spray-dried to obtain a precursor material. The precursor material was incubated in air at 500°C for 10 hours, and then held in an argon atmosphere at 900°C for 3 hours to obtain Li 5 FeO 4 . The purity of the obtained lithium ferrite was detected, and the resu...
Embodiment 2
[0059] This embodiment provides a kind of preparation method of lithium ferrite, the steps are as follows:
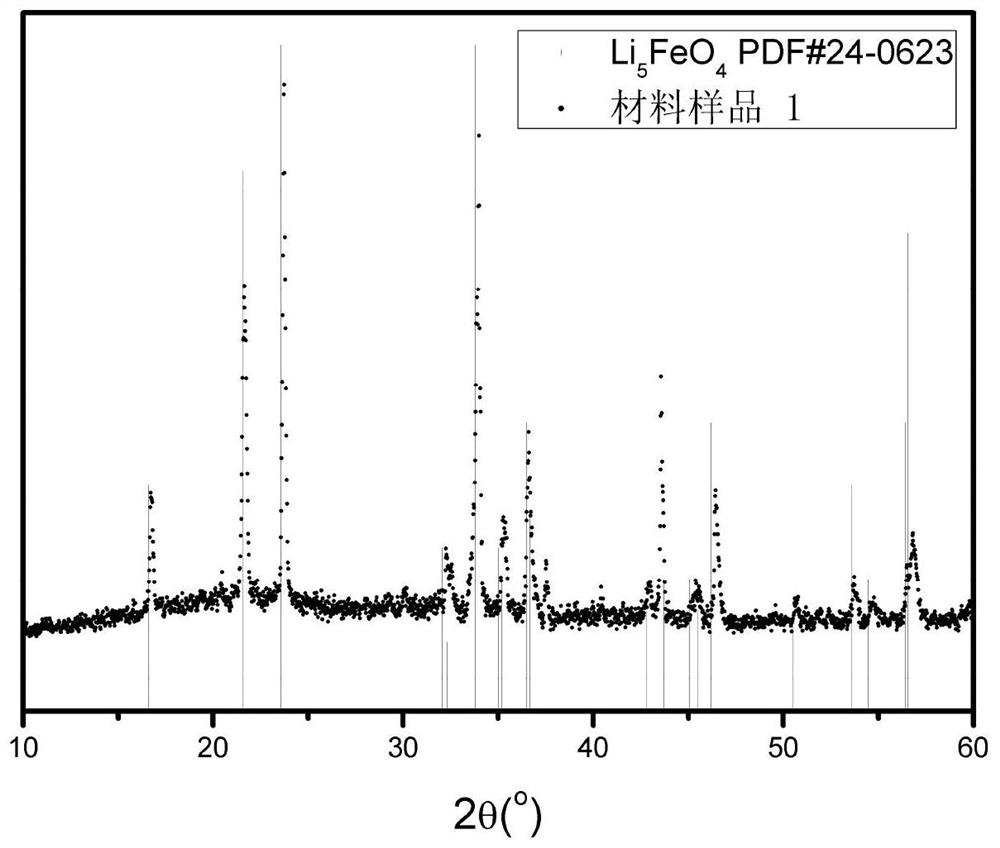
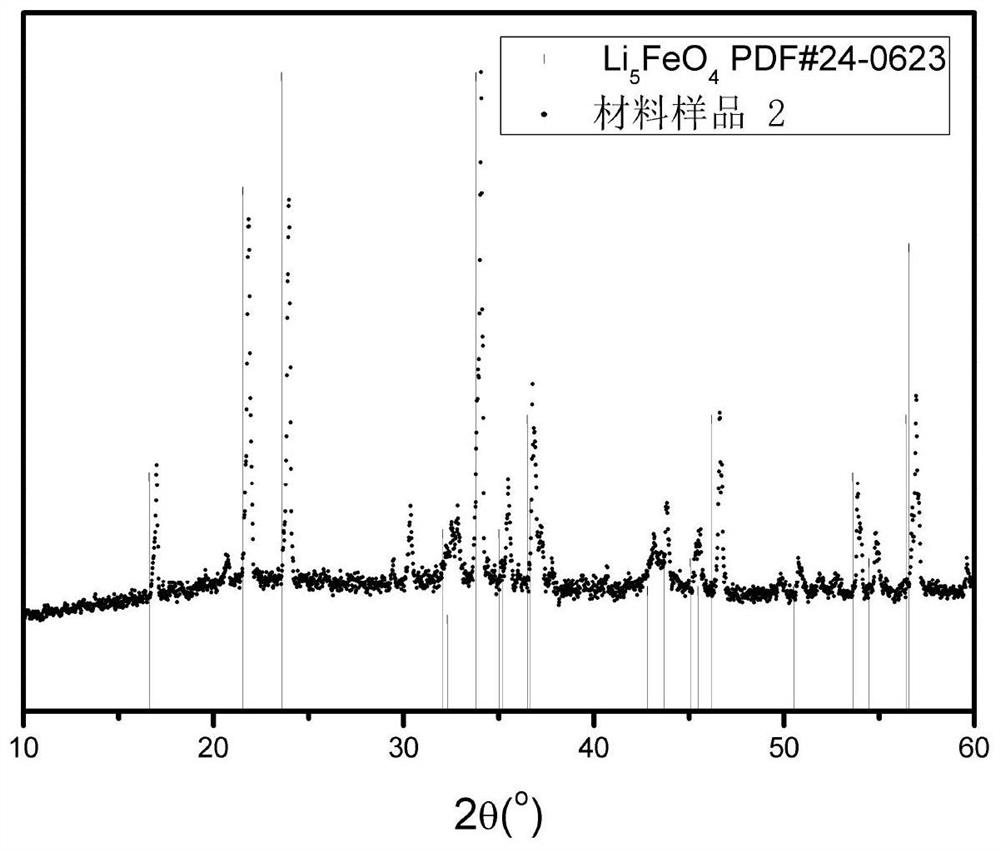
[0060] Fe(NO 3 ) 3 9H 2 O 0.10mol was dissolved in 120mL distilled water, stirred in an ice-water bath, and 2mol / L LiCH 3 COO and 2mol / L Li 2 CO 3 The solution was slowly dropped into the above solution, LiCH 3 The amount of substance added by COO is 0.3mol; Li 2 CO 3 The amount of the substance added is 0.15 mol, and acetic acid is added during the titration process to ensure that the pH is ≤ 3.7. Then put it into a reaction kettle, and perform a solvothermal reaction at 150°C, and the solution after the reaction is spray-dried to obtain a precursor material. The precursor material was incubated in air at 600°C for 6 hours, and then held at 850°C in an argon atmosphere for 6 hours to obtain Li 5 FeO 4 , its XRD data such as figure 1 As shown, its microstructure is as Figure 4 shown. pass figure 1 As can be seen, figure 1 Two characteristic peaks of the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com