Preparation method of recombinant novel coronavirus subunit vaccine
A technology of subunit vaccine and coronavirus, which is applied in the field of preparation of recombinant new coronavirus subunit vaccine, can solve the problems of changing protein structure and lower expression efficiency of recombinant new coronavirus subunit vaccine, and achieves strong immunogenicity and low The effect of cost and high productivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
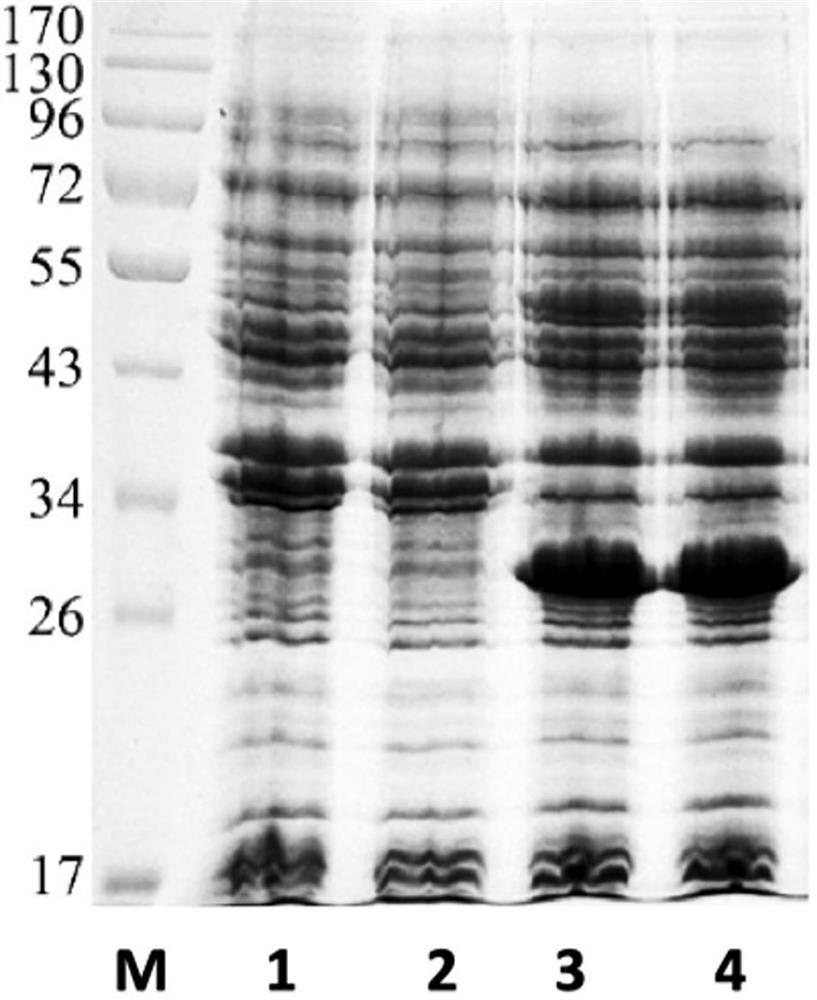
Image
Examples
preparation example Construction
[0030] The present invention relates to a preparation method of a recombinant novel coronavirus subunit vaccine, comprising:
[0031] a) Transfect the plasmid containing the nucleic acid fragment shown in SEQ ID NO: 2 into Escherichia coli, and the nucleic acid fragment can express the RBD-TT fusion protein;
[0032] b) culturing the Escherichia coli to express the RBD-TT fusion protein, disrupting the bacteria and isolating the crude inclusion body extract of the RBD-TT recombinant protein;
[0033] c) dissolving the crude inclusion body extract in a denaturing solution containing urea, and then purifying it by anion exchange chromatography to obtain a crude sample of RBD-TT recombinant protein;
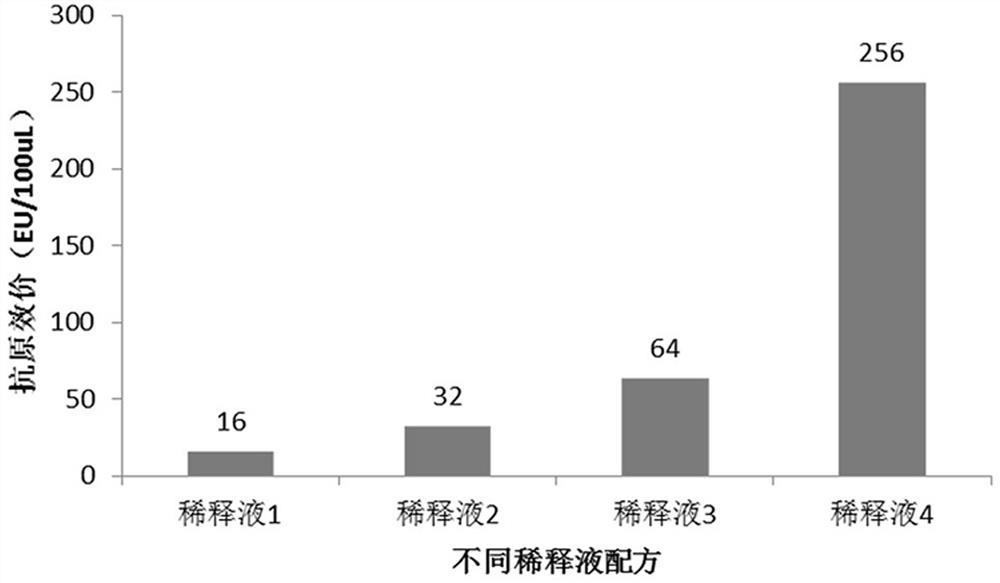
[0034] d) diluting the crude pure sample of the RBD-TT recombinant protein with a diluent;
[0035] Each liter of the diluent contains: 450-510 grams of urea, 70-86 grams of arginine, 0.3-0.7 grams of reduced glutathione, 15-25 grams of glycerin, and buffer substances, pH=9-10;
...
Embodiment 1
[0069] Example 1 Optimization of diluent
[0070] (1) Utilizing the codon merger principle and the codon usage frequency of Escherichia coli, using the amino acid sequence described in SEQ ID NO: 1 as a template, design the nucleotide sequence SEQ ID NO: 2 for expressing the RBD-TT recombinant protein, And the synthesized gene was inserted into pET28a Escherichia coli expression plasmid by the method of seamless cloning to obtain the recombinant expression plasmid. The resulting recombinant expression plasmid was transformed into E. coli BL21 (DE3) competent cells by 42-degree heat activation method, and monoclonal screening was carried out on LB solid medium plates to obtain recombinant E. coli monoclonal colonies expressing the target protein. Escherichia coli monoclonal colonies were cultivated overnight at 37°C with 5 ml of LB liquid medium to obtain recombinant Escherichia coli seed liquid expressing the target protein.
[0071] (2) Take recombinant Escherichia coli seed...
Embodiment 2
[0106] The screening of embodiment 2 recombination conditions
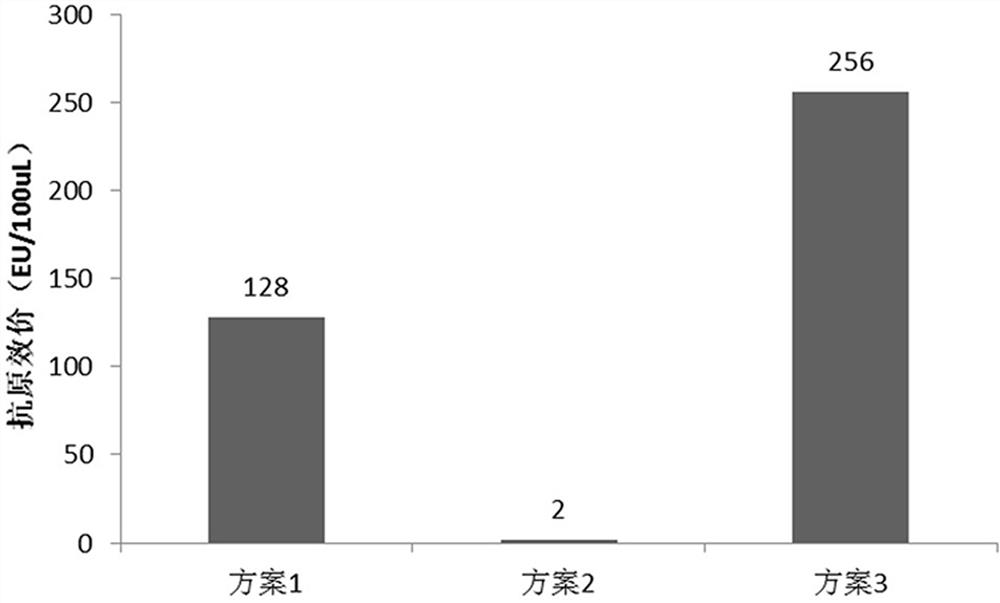
[0107] Operate according to steps (1)~(5) in Example 1, choose diluent 4 as the diluent, and compare the refolding conditions through three schemes: scheme 1, use refolding solution 1 and refolding solution 3 in turn for tangential flow ultrafiltration refolding; plan 2, tangential flow ultrafiltration refolding with refolding solution 2 and refolding solution 3 in sequence; Refolding by directional flow ultrafiltration; the replacement ratio of renaturation liquid and protein liquid used for each tangential flow ultrafiltration renaturation is 1:3, and the feeding pressure of each tangential flow ultrafiltration is 25bar, and the tangential flow ultrafiltration The pore size of the ultrafiltration membrane cassette is 10 kd).
[0108] Take the following step (6):
[0109] (6) The resulting RBD-TT recombinant protein refolding sample was diluted to 0.1 mg / mL, and the antigen titer under different refolding condi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com