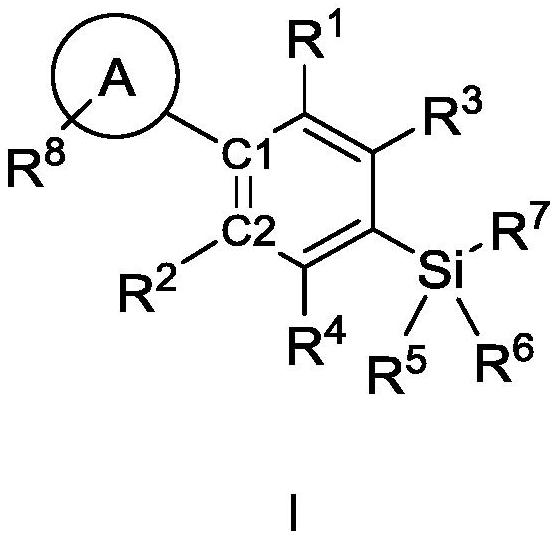
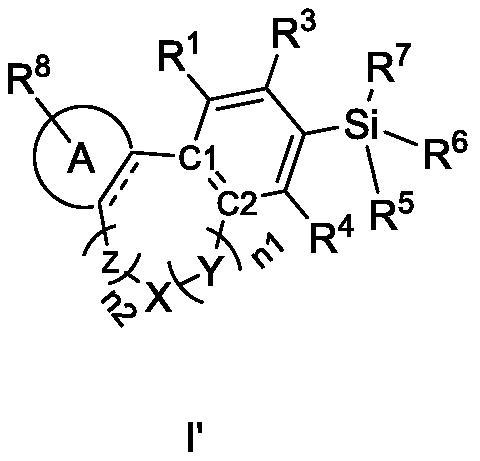
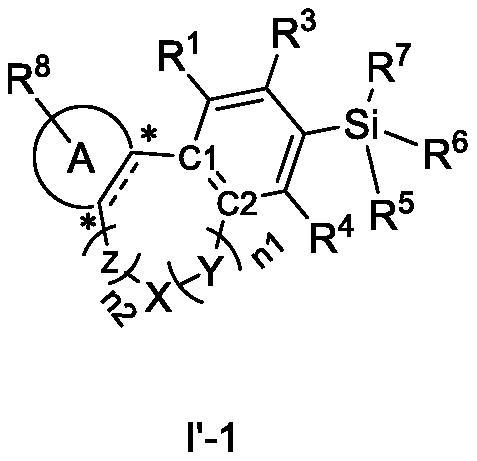
Cannabinoid compound as well as preparation method, composition and application thereof
A technology of compounds and substituents, applied in the field of cannabinoids, can solve problems such as inability to form stable π bonds, affect the effect of drug molecules and receptors, and affect biological effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0147] Example 1: 5-(butyldimethylsilyl)-2-cyclohexyl-3-methoxyphenol (compound 1) and 5-(butyldimethylsilyl)-2-cyclohexylbenzene- Synthesis of 1,3-Diphenol (Compound 2)
[0148] Step 1: Synthesis of Butyl(3,5-Dimethoxyphenyl)dimethylsilane
[0149]
[0150] Weigh 1-bromo-3,5-dimethoxybenzene (500mg, 2.3mmol) into a flask, add anhydrous tetrahydrofuran (10mL) under the protection of argon, cool down to -78°C in the low-temperature reactor, and slowly A solution of n-butyllithium in n-hexane (1.60M, 4.3mL, 6.9mmol) was added dropwise. The reaction was stirred at -78°C for half an hour, after which butyldimethylsilyl chloride (1.04 g, 6.9 mmol) was slowly added dropwise. After stirring for 2 hours, move to room temperature and stir for an additional 1 hour. Add a saturated aqueous solution of ammonium chloride (10 mL) to the reaction system to quench the reaction, extract three times with ethyl acetate, combine the organic phases and wash with water and saturated brine, dr...
Embodiment 2
[0160] Example 2: 5-(hexyldimethylsilyl)-2-cyclohexyl-3-methoxyphenol (compound 3) and 5-(hexyldimethylsilyl)-2-cyclohexylbenzene-1, Synthesis of 3-Diphenol (Compound 4)
[0161] Step 1: Synthesis of (3,5-dimethoxyphenyl)(hexyl)dimethylsilane
[0162]
[0163] Weigh 1-bromo-3,5-dimethoxybenzene (500mg, 2.3mmol) into a flask, add anhydrous tetrahydrofuran (10mL) under the protection of argon, cool down to -78°C in the low-temperature reactor, and slowly A solution of n-butyllithium in n-hexane (1.60M, 4.3 mL, 6.9 mmol) was added dropwise. After the reaction solution was stirred at -78°C for half an hour, hexyldimethylsilyl chloride (1.23 g, 6.9 mmol) was slowly added dropwise, stirring was continued for 2 hours, then moved to room temperature and stirred for another 1 hour. Add a saturated solution of ammonium chloride (10 mL) to the reaction system to quench the reaction, extract three times with ethyl acetate, combine the organic phases and wash with water and saturated ...
Embodiment 3
[0173] Example 3: 5-(Butyldimethylsilyl)-2-cyclopentyl-3-methoxyphenol (compound 5) and 5-(butyldimethylsilyl)-2-cyclopentyl Synthesis of Benzene-1,3-diol (Compound 6)
[0174] Step 1: Synthesis of 1-(4-((butyldimethylsilyl)-2,6-dimethoxyphenyl)cyclopentyl-1-ol
[0175]
[0176] Experimental operation is the same as step 2 of embodiment 1.
[0177] Step 2: Synthesis of butyl(4-cyclopentyl-3,5-dimethoxyphenyl)dimethylsilane
[0178]
[0179] Experimental operation is the same as step 3 of embodiment 1. 1 H NMR (800MHz, deuterated chloroform) δ6.66(s,2H),3.82(s,6H),3.66–3.59(m,1H),1.95–1.86(m,2H),1.86–1.79(m,2H ),1.77–1.72(m,2H),1.65–1.59(m,2H),1.36–1.32(m,4H),0.88(t,J=6.8Hz,3H),0.76–0.73(m,2H), 0.25(s,6H).
[0180] Step 3: 5-(butyldimethylsilyl)-2-cyclopentyl-3-methoxyphenol (compound 5) and 5-(butyldimethylsilyl)-2-cyclopentylbenzene Synthesis of -1,3-diphenol (Compound 6)
[0181]
[0182] Experimental operation is the same as step 4 of embodiment 1. Compound...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com