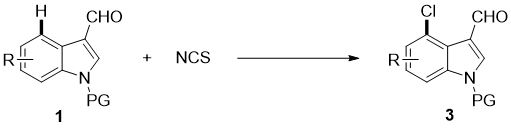4-position chloroindole compound and its preparation method
An indole compound and compound technology, applied in the direction of organic chemistry, can solve problems such as fire, explosion, and difficulty in large-scale application, and achieve the effects of high product purity, good application prospects, and less side reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
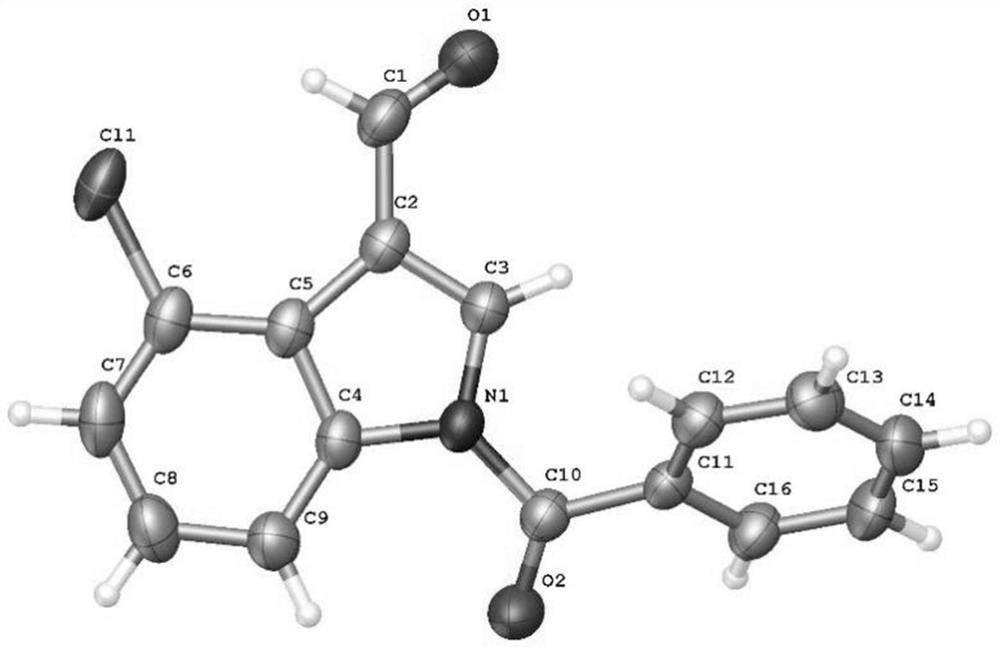
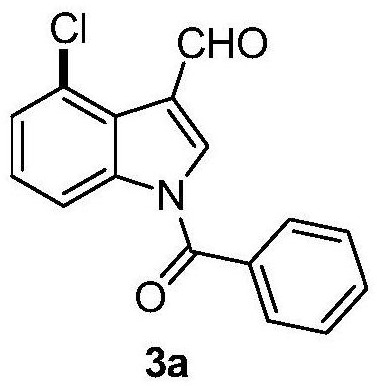
[0054] Add N-benzoylindole-3-carbaldehyde (0.2mmol), N-chlorosuccinimide (0.24mmol), catalyst palladium acetate (10mol%), temporary directing group 2 respectively in the reaction test tube successively. - Amino-4-nitrobenzoic acid (45 mol%), acid additive trifluoroacetic acid (10.0 equiv), and finally the solvent chlorobenzene (1 mL), and the reaction tube was sealed with a rubber stopper. Place the test tube in an oil bath at 50°C and heat it with stirring for about 24 hours. During the reaction, TLC is used to detect that the reaction is complete. During the post-treatment, the solvent was spin-dried first, and the pure product N-benzoyl-4-chloroindole-3-carbaldehyde compound 3a was directly separated by silica gel column chromatography.
[0055]
[0056] Compound 3a, yield: 85%; white solid; melting point 125-127°C; 1 H NMR (400MHz, CDCl 3 )δ10.79(s,1H),8.36(d,J=8.0Hz,1H),8.08(s,1H),7.76–7.74(m,2H),7.69(t,J=7.6Hz,1H), 7.58(t, J=7.6Hz, 2H), 7.44(d, J=7.2Hz, 1H), 7.38(t...
Embodiment 2
[0059] The reactants are N-acetylindole-3-carbaldehyde and N-chlorosuccinimide, and the product is N-acetyl-4-chloroindole-3-carbaldehyde compound 3b.
[0060]
[0061] N-acetyl-4-chloroindole-3-carbaldehyde compound 3b, yield: 80%; white solid; melting point 170-171°C; 1 HNMR (400MHz, CDCl 3 )δ10.79(s,1H),8.43(d,J=8.0Hz,1H),8.21(s,1H),7.39(d,J=8.0Hz,1H),7.33(t,J=8.0Hz, 1H), 2.73(s, 3H); 13 C NMR (100MHz, CDCl 3 )δ187.1, 168.9, 137.4, 130.5, 126.7, 126.0, 125.9, 125.0, 122.2, 115.6, 23.8; HRMS(pos.ESI): m / z[M+H] + forC 11 h 9 ClNO 2 calcd: 222.0316, found: 222.0351.
Embodiment 3
[0063] The reactants are N-benzyloxycarbonyl indole-3-carbaldehyde and N-chlorosuccinimide, and the product is N-benzyloxycarbonyl-4-chloroindole-3-carbaldehyde compound 3c.
[0064]
[0065] N-benzyloxycarbonyl-4-chloroindole-3-carbaldehyde compound 3c, yield: 46%; white solid; melting point 93-95°C; 1 HNMR (400MHz, CDCl 3 )δ10.78(s,1H),8.42(s,1H),8.23(d,J=8.0Hz,1H),7.50–7.43(m,5H),7.39(d,J=8.0Hz,1H), 7.33(t,J=8.0Hz,1H),5.49(s,2H); 13 C NMR (100MHz, CDCl 3 )δ186.9,149.8,137.2,134.0,131.2,129.3,129.0,128.9,126.3,126.2,125.4,125.1,121.8,114.3,70.1; HRMS(pos.ESI):m / z[M+H] + for C 17 h 13 ClNO 3 calcd: 314.0578, found: 314.0624.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com