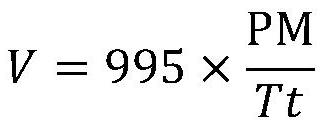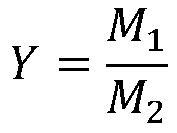Method for continuously preparing nitrile from amide
A technology of amide and amide, which is applied in the field of continuous preparation of nitriles from amides, can solve the problems of continuous production, corrosion of equipment, environmental protection pressure, and ineffective reaction, etc., to achieve wide substrate applicability, reduce three wastes discharge, The effect of low thermal stability requirement
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Example 1. A method for the efficient dehydration of benzamide and continuous preparation of benzonitrile catalyzed by a molecular sieve-supported lead catalyst
[0034] (1) immerse 100g molecular sieve in the aqueous solution 100ml that contains 10g lead diacetate, until this solution is completely absorbed by molecular sieve;
[0035] The obtained impregnated molecular sieve was dried in a drying oven at 80° C. for 2 hours, at 120° C. for 2 hours, and then calcined in a muffle furnace at 400° C. for 2 hours to obtain a catalyst.
[0036] In this catalyst, the atomic loading of lead is 6.4 wt% of the molecular sieve.
[0037] (2) Fill the above-mentioned catalyst in a fixed-bed reactor.
[0038] The height of the fixed bed reactor is L=1500mm, the inner diameter D=100mm; 5000g of catalyst is installed inside.
[0039] The top of the fixed-bed reactor is equipped with a pre-heater, the set temperature of the pre-heater is 150°C, and the reaction temperature (bed tempe...
Embodiment 2~8
[0055] Examples 2-8, changing the load of lead diacetate, other operations are the same as in Example 1, to obtain Examples 2-8, see Table 1 for process parameters and reaction results.
[0056] Table 1
[0057] Example Loading amount of lead diacetate (wt%) Raw material conversion rate (%) Reaction selectivity (%) Product yield (%) 2 2 91.3 100 88.7 3 5 94.6 100 92.2 4 8 95.9 100 93.5 5 12 97.5 100 95.5 6 15 97.7 100 95.7 7 18 97.9 100 95.8 8 20 98.0 100 95.8
Embodiment 9~13
[0058] Examples 9-13, changing the type of lead salt, the atomic loading of lead remains unchanged, which is still 6.4wt% of the molecular sieve. Other operations were the same as in Example 1 to obtain Examples 9-13. See Table 2 for process parameters and reaction results.
[0059] Table 2
[0060] Example lead salt Raw material conversion rate (%) Reaction selectivity (%) Product yield (%) 9 Lead metaborate 97.0 100 94.8 10 lead chloride 93.7 100 91.2 11 lead bromide 94.1 100 92.0 12 lead phosphate 96.5 100 94.3 13 lead nitrate 95.2 100 92.8
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com