Antiretroviral pharmaceutical composition and preparation method thereof
An anti-retrovirus and reverse transcriptase inhibition technology, which is applied in antiviral agents, pharmaceutical formulas, sugar-coated pills, etc., can solve the problems of small drug loading and easy aging, and achieve low content of related substances, good stability, The effect of improving wettability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] The present invention provides an antiretroviral pharmaceutical composition, which comprises nucleoside reverse transcriptase inhibitors and integrase inhibitors and one or more pharmaceutically acceptable excipients, wherein the The weight ratio of nucleoside reverse transcriptase inhibitor and integrase inhibitor is 4: 1; Described nucleoside reverse transcriptase inhibitor (NRTI), it comprises lamivudine, abacavir, Zidov One or more combinations of Ding, Emtricitabine, Didanosine, Stavudine, Entecavir, Zalcitabine, dexelvucitabine, elvucitabine or their pharmaceutically acceptable derivatives or their mixtures, this application Emtricitabine or a pharmaceutically acceptable derivative thereof or a mixture thereof is preferred; the integrase inhibitor comprising dolutegravir, elvitegravir, raltegravir, bictegravir, cabotegravir or a pharmaceutically acceptable derivative thereof or a mixture thereof One or more combinations, preferably dolutegravir or its pharmaceutic...
Embodiment 2
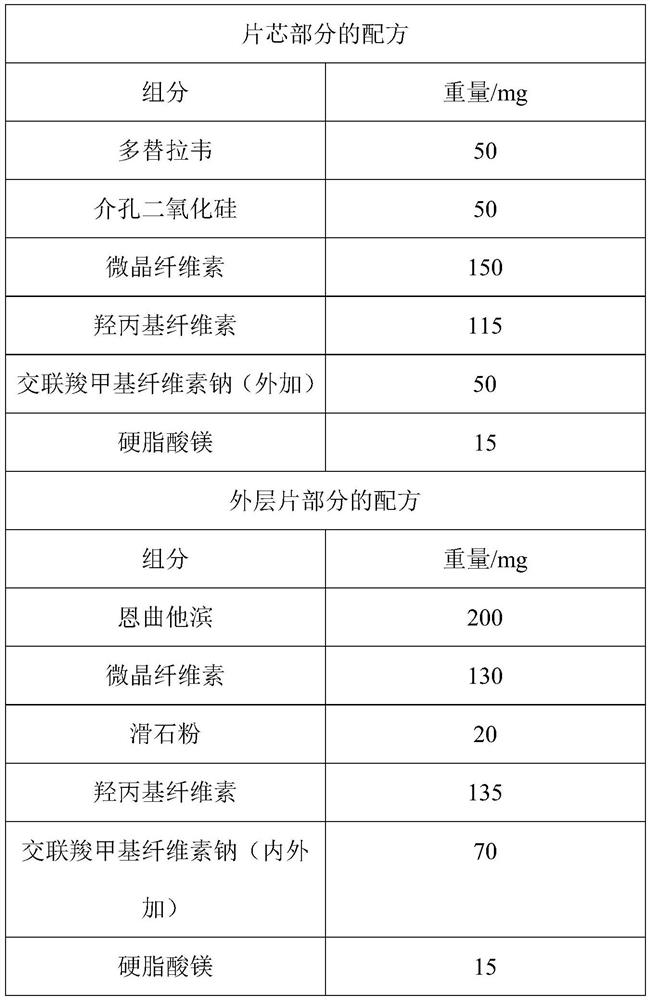
[0049] Prescription: as in Table 1:
[0050] Table 1 The formula of the compound package chip of the present invention
[0051]
[0052] A preparation method of an antiretroviral compound package chip is as follows:
[0053] (a) Preparation of tablet core part:
[0054] (a1) 50 mg of mesoporous silica was ultrasonically treated at 25°C for 30 minutes by ultrasonic oscillation technology, and prepared into a suspension with a mass concentration of 13%. The suspension was treated under mm conditions for 4 minutes, then centrifuged at 2500 rpm for 13 minutes to obtain a precipitate, washed 4 times with absolute ethanol, and vacuum freeze-dried to obtain modified mesoporous silica.
[0055] (a2) Dissolve the modified mesoporous silica obtained in step (a1) and 50 mg of dolutegravir in an appropriate amount of absolute ethanol, and perform ultrasonic treatment at intervals of 2 seconds at a frequency of 18KHz and a power of 350W, with an interval of 4 seconds , the total time...
Embodiment 3
[0068] Prescription: as shown in Table 2
[0069] Table 2 The formula of the compound package chip of the present invention
[0070]
[0071]
[0072] A preparation method of an antiretroviral compound package chip is as follows:
[0073] (a) Preparation of tablet core part:
[0074] (a1) 50 mg of mesoporous silica was ultrasonically treated at 30°C for 40 minutes by ultrasonic oscillation technology, and prepared into a suspension with a mass concentration of 15%, and directly used an air atmospheric pressure plasma jet device with a power of 850W and a height of 14.13 The suspension was treated under mm conditions for 7 min, and then centrifuged at 3000 rpm for 10 min to obtain a precipitate, washed 5 times with absolute ethanol, and vacuum freeze-dried to obtain modified mesoporous silica.
[0075] (a2) Dissolve the modified mesoporous silica obtained in step (a1) and 50 mg of dolutegravir in an appropriate amount of absolute ethanol, and perform ultrasonic treatmen...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com