Doramectin sustained-release microcapsule injection
A technology of doramectin and injection, which is applied in the field of antiparasitic pharmaceutical preparations, and can solve problems such as short half-life, impact on the healthy growth of livestock, and stimulation response of livestock and poultry
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
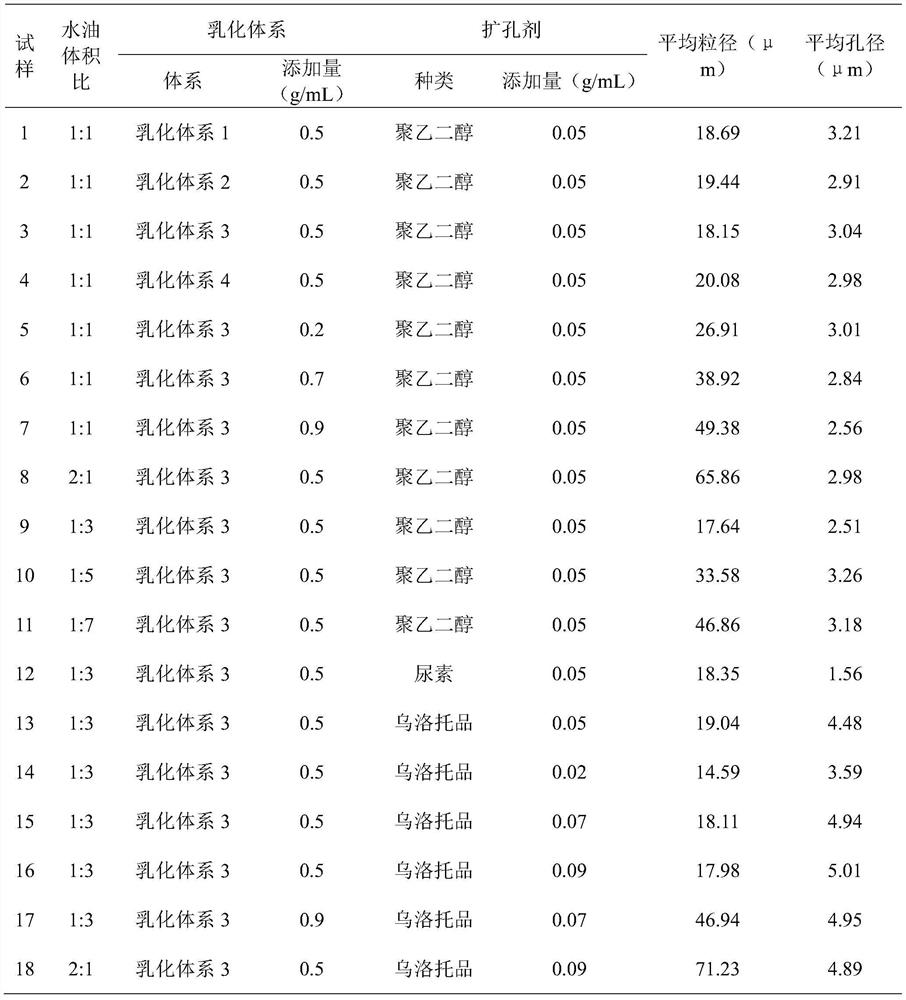
[0031] 1. Preparation of silica airgel particles:
[0032] (a) Add tetraethyl orthosilicate to deionized water, slowly add ethanol until the mixed solution becomes clear;
[0033] (b) Adding dilute hydrochloric acid under stirring to adjust the pH to 3.0-4.0, standing still at room temperature for 12-24 hours, adding a pore-enlarging agent to prepare an aqueous phase system;
[0034] (c) Use n-hexane as the oil phase, mix the emulsification system and stir evenly to make an oil phase system, add the water phase system of step (b) into the oil phase system under stirring, add ammonia water under stirring to adjust the pH to 7.0 ~8.0 and continue to stir for 15 minutes, let it stand until the wet gel is formed, add the protective solution and put it in a closed container for aging;
[0035] (d) add n-hexane to soak in the wet gel after aging, perform solvent exchange, and replace n-hexane several times;
[0036] (e) removing solvent to obtain silica airgel powder;
[0037] (f...
Embodiment 1
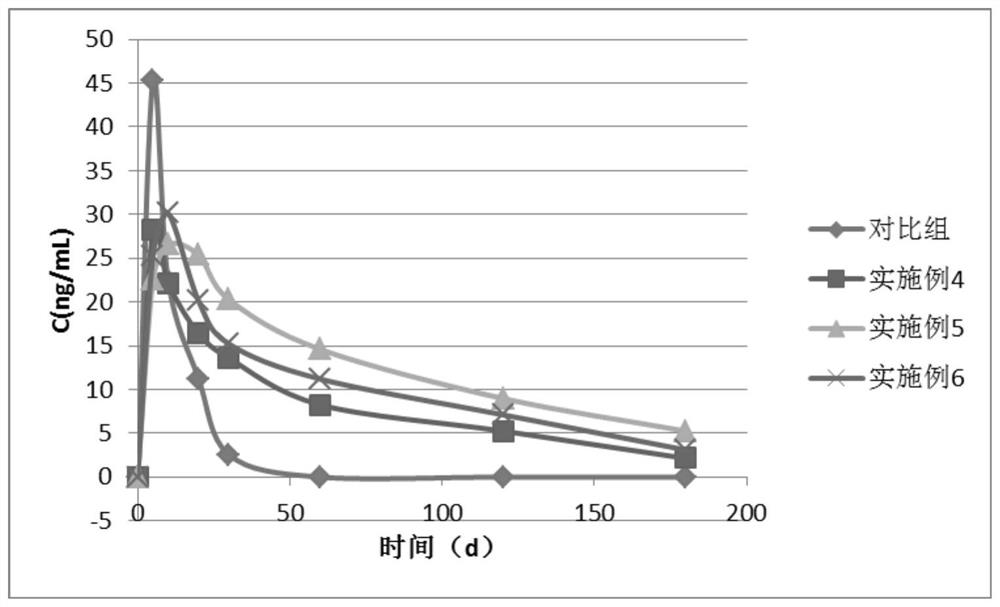
[0060] (1) Dissolve poly-L-lactic acid and poly-D-lactic acid with a molecular weight of 1-10W in chloroform to obtain 10g / L poly-L-lactic acid solution and 10g / L poly-D-lactic acid solution, and then add excess doramectin, stirred and filtered to obtain poly L-lactic acid solution and poly D-lactic acid solution containing saturated doramectin.
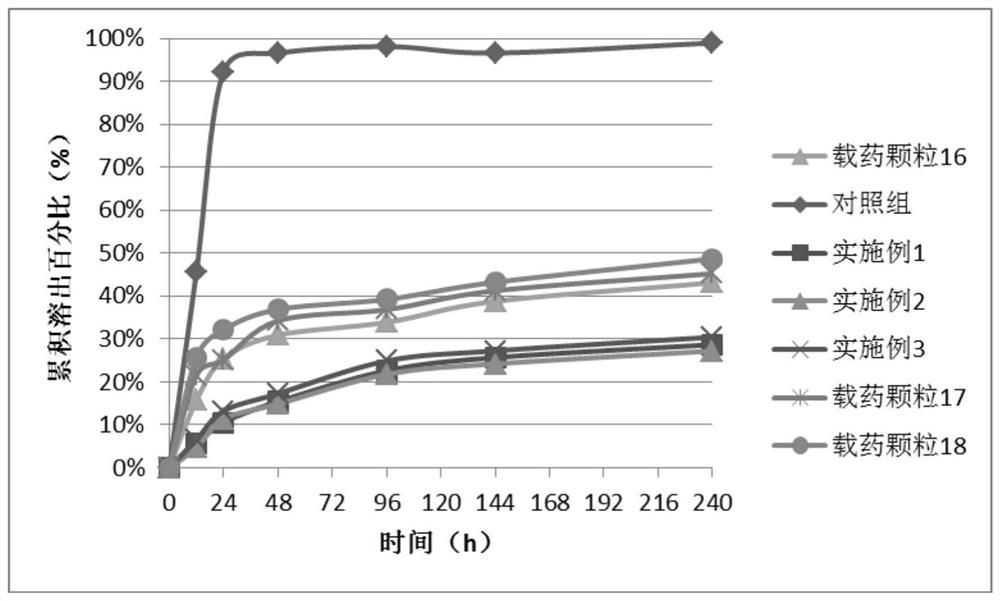
[0061] (2) Add the drug-loaded particles 16 into a centrifuge tube, add poly-L-lactic acid solution and shake to disperse the particles, shake at 40°C for 1 hour, and wash with chloroform centrifuge for 3 times, the centrifugation parameters are 1000r / min, 1min;
[0062] (3) Add poly-D-lactic acid solution into the centrifuge tube and oscillate to disperse the particles, oscillate at 40°C for 1 hour, wash with chloroform for 3 times, and the centrifugation parameter is 1000r / min, 1min;
[0063] (4) Freeze-dry to constant weight to obtain polylactic acid-wrapped silicon dioxide airgel-doramectin microcapsule particles.
[0064] Compa...
Embodiment 2
[0066] (1) Dissolve poly-L-lactic acid and poly-D-lactic acid with a molecular weight of 1-10W in chloroform to obtain 10g / L poly-L-lactic acid solution and 10g / L poly-D-lactic acid solution, and then add excess doramectin, stirred and filtered to obtain poly L-lactic acid solution and poly D-lactic acid solution containing saturated doramectin.
[0067] (2) Add the drug-loaded particles 17 into a centrifuge tube, add poly-L-lactic acid solution and oscillate to disperse the particles, oscillate at 40°C for 1 hour, and centrifuge and wash 3 times with chloroform, the centrifugation parameter is 1000r / min, 1min;
[0068] (3) Add poly-D-lactic acid solution into the centrifuge tube and oscillate to disperse the particles, oscillate at 40°C for 1 hour, wash with chloroform for 3 times, and the centrifugation parameter is 1000r / min, 1min;
[0069] (4) Freeze-dry to constant weight to obtain polylactic acid-wrapped silicon dioxide airgel-doramectin microcapsule particles.
[0070]...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com