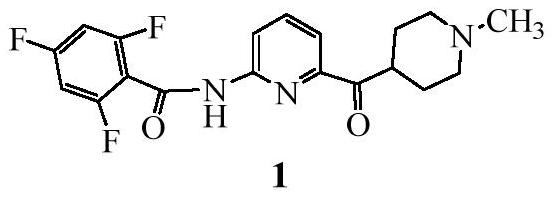
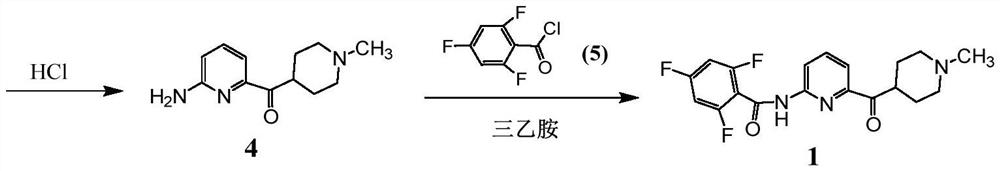
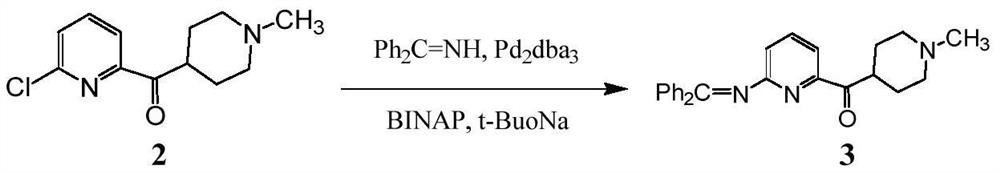Synthetic process of Lasmiditan
A synthesis process, bromopyridine technology, applied in the direction of organic compound/hydride/coordination complex catalyst, catalytic reaction, organic chemistry, etc., can solve the problems of only 37% yield and too expensive
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0081] Preparation of 2,4,6-trifluorobenzamide
[0082] 175g (1.00mol) of 2,4,6-trifluorobenzoic acid and 429g (261mL, 3.60mol) of thionyl chloride were refluxed for 3 hours, and most of the excess thionyl chloride was distilled off. Add the reaction materials to 200mL of 28% ammonia water, control the ammoniation reaction temperature to be less than 5°C, after the addition is complete, stir and react for 2h, filter with suction, wash with cold water, and dry to obtain 2,4,6-trifluorobenzamide (hereinafter Abbreviation: trifluorobenzamide) light yellow solid 160g, yield 91.0%; 1 H NMR (600M Hz, DMSO-d 6 ): δ8.14(s,1H),7.88(s,1H),7.26(dd,J=9.1 / 8.0Hz,2H).
[0083] Purity: 99.51% (HPLC conditions: chromatographic column Gemini 5μC18 110A, 250mm×4.6mm, acetonitrile:water=12:88, pH 5, λ230nm, flow rate 1.0ml / mL, R t 11.89min).
[0084] Preparation of Lasmiditan
[0085] Add anhydrous potassium carbonate 19.4g (141mmol), trifluorobenzamide 9.0g (51mmol), cuprous iodide 887mg (...
Embodiment 2
[0087] Example 2 is the same as Example 1, except that the consumption of cuprous iodide is halved to 444mg (2.33mmol)
[0088] Add 19.4 g of anhydrous potassium carbonate, 9.0 g of trifluorobenzamide, and 444 mg (2.33 mmol) of cuprous iodide into the reaction flask, remove the air, add 300 mL of tetrahydrofuran, ligand—N,N’-dimethyl-1 , 0.99 mL of 2-ethylenediamine, 13.2 g of bromopyridone, and the mixture was stirred and reacted at room temperature for about 24 hours. The reaction material was filtered, and the solid was washed with tetrahydrofuran, and then the filtrate was washed with 50 mL of 0.10M sodium sulfide solution, 50 mL of water, and then 50 mL of 0.20 M hydrochloric acid to wash away the ethylenediamine ligands, and then lasmiditan was washed with 100 mL of 0.15 M hydrochloric acid. Salt is dissolved in water, the aqueous solution containing product hydrochloride is decolorized with activated carbon, the aqueous solution is basified with 5M NaOH solution 3.4mL t...
Embodiment 3
[0089] Example 3 is the same as Example 1, except that the amount of cuprous iodide is doubled to 1.74g (9.32mmol)
[0090] Add 19.4g of anhydrous potassium carbonate, 9.0g of trifluorobenzamide, 1.74g (9.32mmol) of cuprous iodide to the reaction flask, remove the air, add 300mL of tetrahydrofuran, ligand—N,N'-dimethyl- 0.99 mL of 1,2-ethylenediamine, 13.2 g of bromopyridone, and the mixture was stirred and reacted at room temperature for about 24 hours. The reaction material was filtered, and the solid was washed with tetrahydrofuran, and then the filtrate was washed with 50 mL of 0.10M sodium sulfide solution, 50 mL of water, and then 50 mL of 0.20 M hydrochloric acid to wash away the ethylenediamine ligands, and then lasmiditan was washed with 100 mL of 0.15 M hydrochloric acid. Salt is dissolved in water, the aqueous solution containing product hydrochloride is decolorized with activated carbon, the aqueous solution is basified with 5M NaOH solution 3.4mL to precipitate lasm...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com