Method for preparing fluorescent probe with tumor targeting effect by taking ferritin as carrier
A technology of ferritin and carrier, which is applied in the field of preparation of fluorescent probes and fluorescent probes, can solve the problems of poor solubility of coumarin-6, etc., and achieve the effect of uniform particle size, high utilization rate and small particle size
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] E. coli expressing the protein of interest was inoculated into LB medium containing ampicillin, 37 [deg.] C temperature, 210r / min shaking cultured to an OD600 of between 0.6 to 0.8, IPTG was added to the cultures to a final concentration of 0.5 mM, the culture was 30 ℃, 180r / min continued induced culture 6-8h. The bacteria 8000r / min 5min to collect the cells after centrifugation, the cells were dissolved in lysis buffer (50mM NaH 2 PO 4 , 200mM NaCl, 10mM imidazole, pH = 7.9) for hanging weight. Suspension was broken at intervals of 5s after 15min, 8000r / min centrifugal supernatant was collected 5min, 10min the supernatant was heat-treated in water bath 60 ℃. The treated crude protein centrifuged at 8000r / min 30min the supernatant was collected, and the supernatant bound 6-12h nickel column, after elution through imidazol 5mM, 10mM, 250mM ferritin obtained crude sample, using an FPLC to give more pure HFtn .
Embodiment 2
[0031] 1:200 proportion (molar ratio) was weighed ultrasonic coumarin-6 was dissolved in absolute ethanol. The coumarin-6 was dissolved ferritin mixture was stirred at 4 ℃ 5min, mixed thoroughly. Using a mixed solution of 6M HCl solution the pH of the protein solution was adjusted to 2.5, were mixed and stirred at 4 ℃ 30min, depolymerization -6 sufficient contact with coumarin ferritin subunits. The mixing is complete the solution of 6M NaOH solution adjusted to pH 7.2, mixed and stirred at 4 ℃ 2h, depolymerization ferritin reassembled into the cage structure.
[0032] The protein solution using pH = PBS buffer 7.4 8-12h dialysis at 4 ℃, to remove non-entrapped coumarin-6. The dialyzed solution was 5000r / min centrifugal 10min, to remove the denatured proteins, it will give COU-HFtn nanoprobe solution.
[0033] HFtn-COU nanoprobes obtained in this embodiment, wherein the content of the coumarin-6 ferritin 1:200 ratio (molar mass ratio)
Embodiment 3
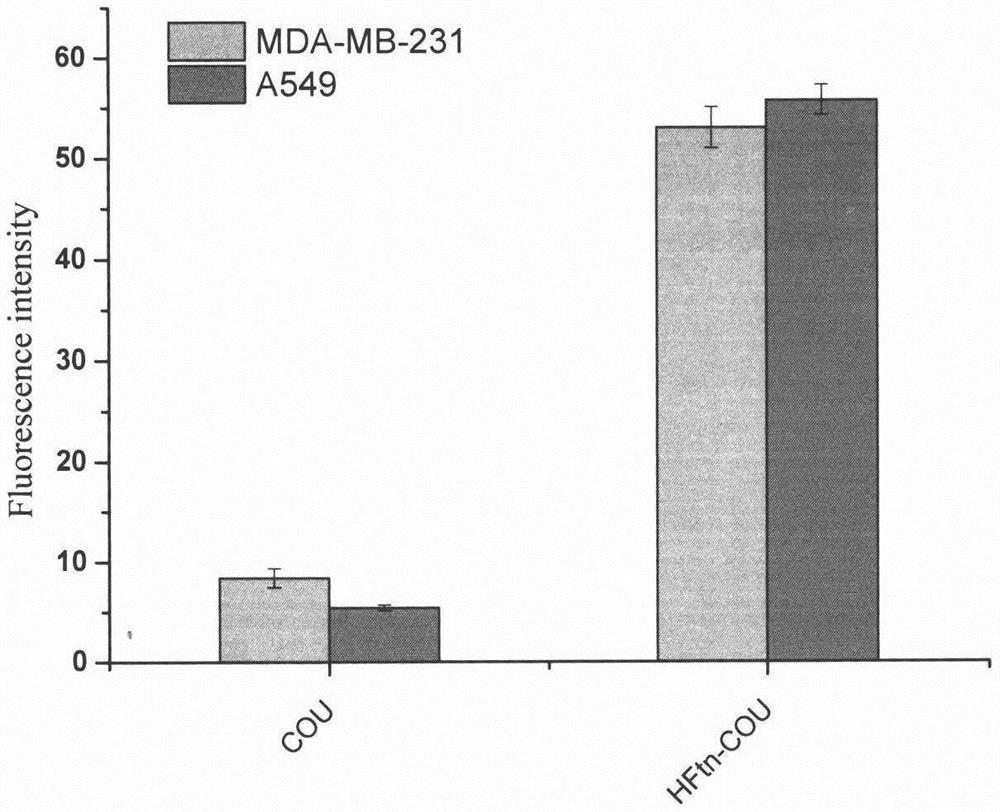
[0035] Fluorescence intensity HFtn-COU nanoparticles Analysis
[0036] (1) Take 1 × 10 4 The concentration of cells per well is inoculated with 24-well plate, 100 μL per well; at 37 ° C, 5% CO 2 The culture tank was cultured 24h to make cellular wall.
[0037] (2) 100 μl of the medium containing 0.5 μg / ml of COU and HFTN-COU is added to each well, each need to be repeated, and all solutions are added to 37 ° C, 5% CO. 2 Culture in the incubator for 24 h.
[0038] (3) After 24 hours of incubation, carefully suck all the supernatings in the well, rinse three times using the PBS buffer, wash away the residual medium. The cells were digested using trypsin and centrifuged and dissolved in 200 μl of DMSO solution after centrifugation.
[0039] (4) Determination of fluorescence intensity, recording data is detected using the enzymatic wavelength of 456 nm, and the emission wavelength is 504 nm, and the data is detected, and the drug name is the abscissa, the fluorescence intensity is a l...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com