Use of aescin in the preparation of medicines for treating ulcerative colitis
A technology for ulcerative colitis and escin, which is applied in the field of escin in the field of preparing medicines for treating ulcerative colitis, can solve problems such as immune inflammation such as UC that have not yet been found, and achieve the effects of significant symptoms, symptom prevention and treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Detection of anti-ulcerative colitis drug components of the present invention
[0030] 1. Experimental method
[0031] The content of the main component of the aescin drug is determined, and HPLC is used for detection, and the chromatographic conditions are as follows:
[0032] Chromatographic column: Agilent 5HC-C18, 4.6*250mm, 5.0μm;
[0033] Column temperature: 30°C;
[0034] UV detection wavelength: 220nm;
[0035] Flow rate: 1.0ml / min;
[0036] Mobile phase: A, 0.1% phosphoric acid aqueous solution; B, acetonitrile;
[0037] Isocratic elution: B: 42%.
[0038] 2. Experimental results
[0039] The HPLC chromatograms of the main components are shown in figure 1 and content see figure 2 . The HPLC chromatogram of aescin and the chromatographic peak information table of each component are shown in the table. Among them, the content of the main component aescin A in aescin accounts for 30.42% of the total content, the content of aescin B accounts for 15.38% o...
Embodiment 2
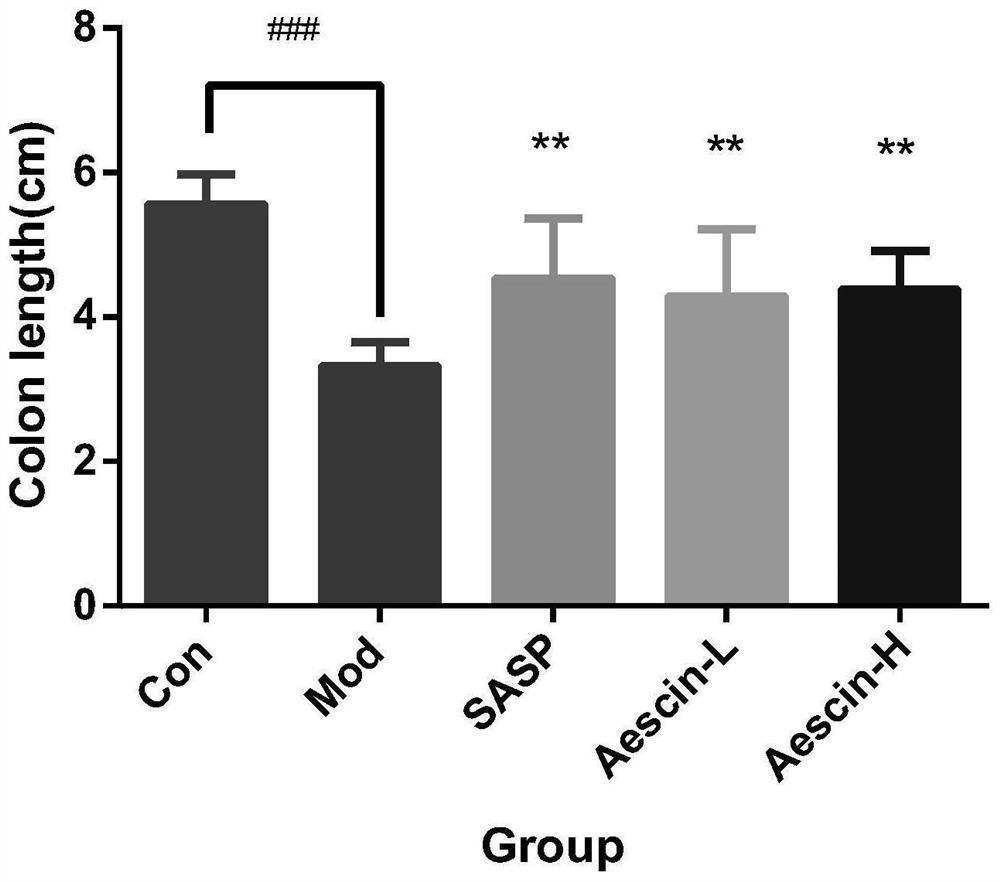
[0041] Effect of anti-ulcerative colitis drug of the present invention on mouse UC model
[0042] 1. Test materials
[0043] 1.1 Drugs and dosage
[0044] The experimental drugs include one test drug and sulfasalazine as the positive control drug, and the specific dosage is shown in Table 2
[0045] Table 2. Tested drugs and dosage
[0046]
[0047] 1.2 Test animals
[0048] C57 mice, male, Center for Comparative Medicine, Yangzhou University, license number: SCXK (Su) 2017-0007.
[0049] 1.3 Test equipment
[0050] Ultrapure water preparation system; ordinary refrigerator; ultra-low temperature refrigerator; ice crusher; electronic balance.
[0051] 1.4 Solution preparation
[0052] (1) 2.5% DSS (w / v) solution: weigh 2.5g DSS powder, dissolve it in 100ml deionized water, and fully dissolve until the system is transparent.
[0053] (2) 0.01M PBS solution: weigh 0.2g KCl, 7.9g NaCl, 1.44g Na 2 HPO 4 and 1.8g K 2 HPO 4 , dissolved in 800ml of deionized water, adjus...
Embodiment 3
[0075] Aescin 100 mg, mixed with 50 mg of starch and 50 mg of dextrin, made into a soft material with an appropriate amount of 30% ethanol as a wetting agent, granulated by conventional methods, mixed with magnesium stearate, and made into tablets.
[0076] 50 mg of aescin is mixed with 70 mg of starch, 10 mg of dextrin, and 10 mg of powdered sugar, and 30% ethanol is used as a wetting agent to make a soft material, which is prepared into granules by wet granulation.
[0077] Aescin 100mg is mixed with starch 50mg and talcum powder 5mg to make powder by conventional technology.
[0078] 100 mg of aescin, 20 mg of powdered sugar and 5 mg of water are prepared into a syrup by conventional technology, and then 70 mg of starch is added to prepare a pill by conventional technology.
[0079] Aescin 100g is decocted twice with 1000g water by traditional decoction method, and the decoction is combined to make a decoction.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com