Phenylalanine dehydrogenase as well as preparation method and application thereof
A technology of phenylalanine dehydrogenase and its use, which is applied in the field of extreme amino acid dehydrogenase and its preparation, and the ability to catalyze unnatural amino acids, and can solve the problems of less attention to stress resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Embodiment 1: the preparation of phenylalanine dehydrogenase
[0036] 1) Preparation of a recombinant expression strain expressing the phenylalanine dehydrogenase: according to the nucleotide sequence shown in SEQ ID NO.2, design the upstream primer shown in SEQ ID NO.3 and the sequence shown in SEQ ID NO. The downstream primer shown in NO.4, and construct the phenylalanine dehydrogenase gene sequence with NdeI and Xhol restriction sites at the 5' end and 3' end respectively by PCR method, double enzyme digestion and connection with the vector pET28a , to obtain the pET28a-PheDH plasmid; transform the pET28a-PheDH plasmid into E.coliBL21(DE3), and obtain the recombinant expression strain E.coli BL21(DE3) that can express the phenylalanine dehydrogenase with the His-tag tag / pET28a.
[0037] 2) Cultivation of the recombinant expression strain E.coli BL21(DE3) / pET28a: Inoculate the recombinant expression strain E.coli BL21(DE3) / pET28a into 200 mL of LB medium at an inocu...
Embodiment 2
[0041] Embodiment 2: The influence of 20% organic solvent on phenylalanine dehydrogenase
[0042] 1) Preparation and cultivation of the recombinant expression strain: the same as Step 1)-2) of Example 1;
[0043] 2) Preparation of crude enzyme solution and pure enzyme: same as step 3) to 4) of Example 1;
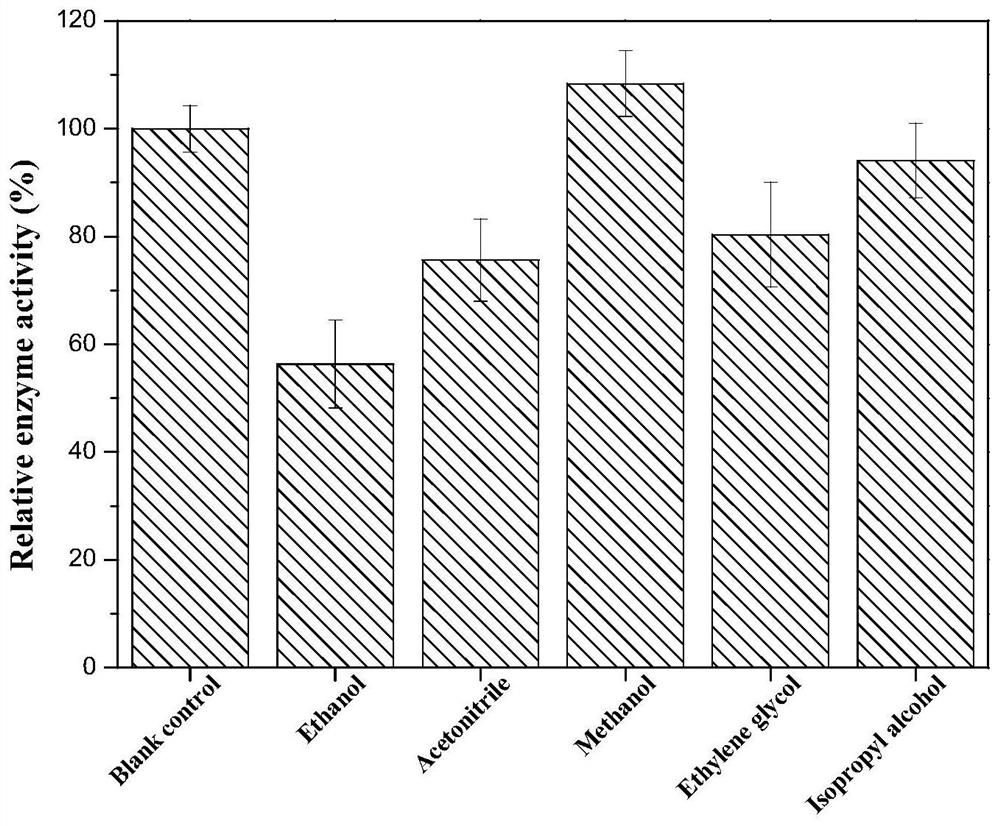
[0044] 3) Determination of the influence of organic solvents on enzyme activity: the substrates 2-oxo-4-phenylbutyric acid ethyl ester and NADH were dissolved in the glycine-sodium hydroxide buffer solution of pH 9, the concentration of the substrate was 10mM, phenylpropanol The concentration of amino acid dehydrogenase is 20U / L. Under parallel conditions, add different organic solvents (ethanol, acetonitrile, methanol, ethylene glycol, isopropanol, etc.) Reaction, determination of enzyme activity, so as to determine the impact of different organic solvents on phenylalanine dehydrogenase, to add an equal amount of 0.05mol / L NH 4 Cl-NH 3 ·H 2 O (pH 8) was used as a contr...
Embodiment 3
[0046] Embodiment 3: The influence of 30% organic solvent on phenylalanine dehydrogenase
[0047] 1) Preparation and cultivation of the recombinant expression strain: the same as Step 1)-2) of Example 1;
[0048] 2) Preparation of crude enzyme solution and pure enzyme: same as step 3) to 4) of Example 1;
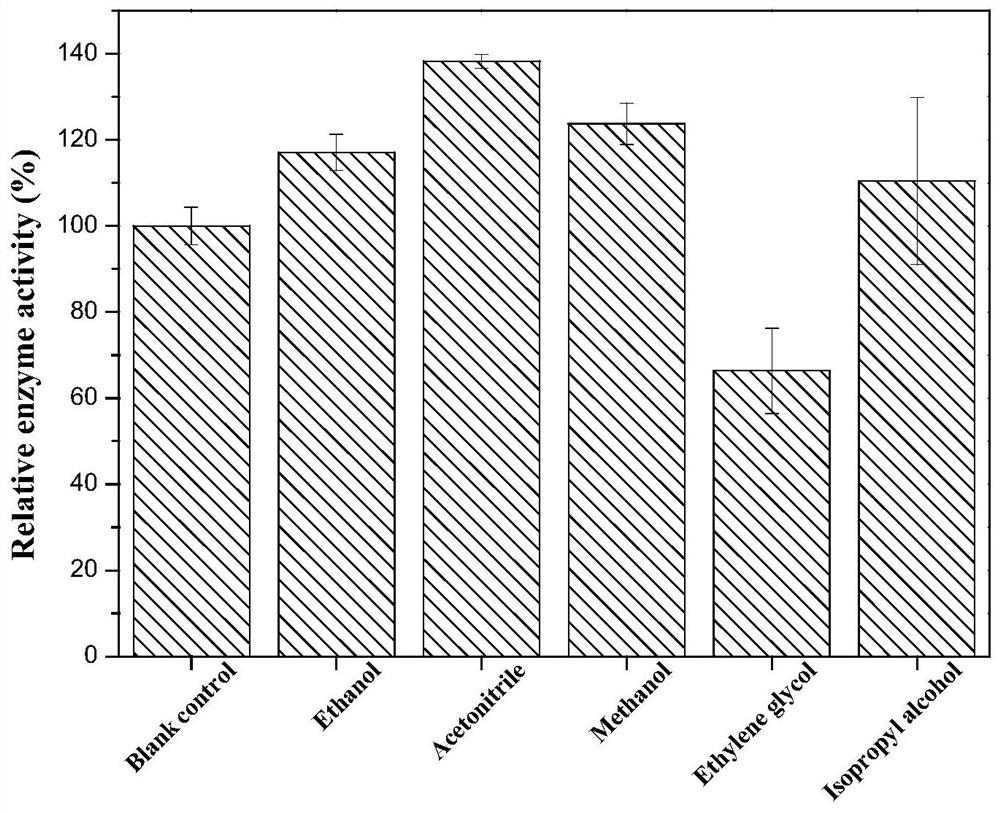
[0049] 3) Determination of the influence of organic solvents on enzyme activity: the substrates 2-oxo-4-phenylbutyric acid ethyl ester and NADH were dissolved in the glycine-sodium hydroxide buffer solution of pH 10, the concentration of the substrate was 100mM, phenylpropanol Amino acid dehydrogenase concentration is 50U / L. Under parallel conditions, add different organic solvents (ethanol, acetonitrile, methanol, ethylene glycol, isopropanol, etc.) reaction, to determine the enzyme activity, so as to determine the influence of different organic solvents on phenylalanine dehydrogenase, to add an equal amount of 0.15mol / L NH 4 Cl-NH 3 ·H 2 O (pH 10.5) was used as a cont...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com