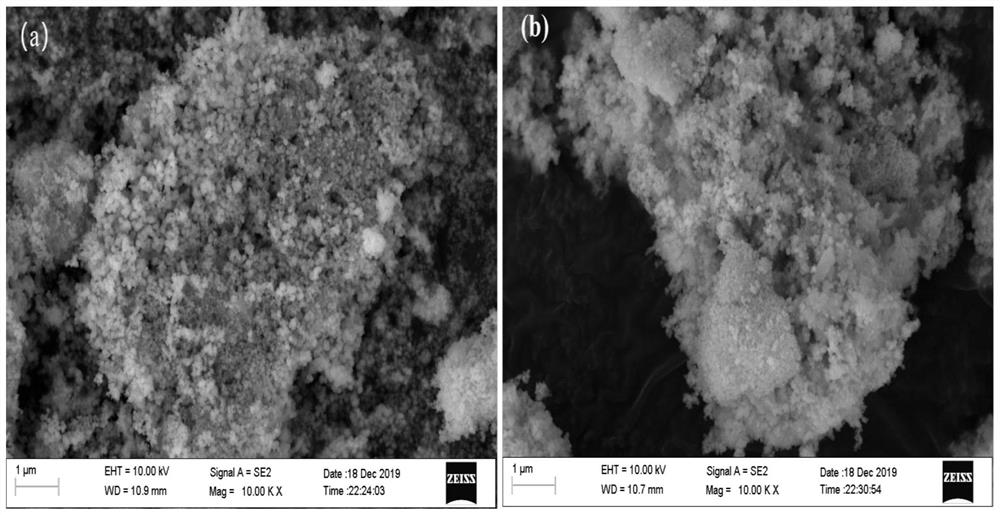
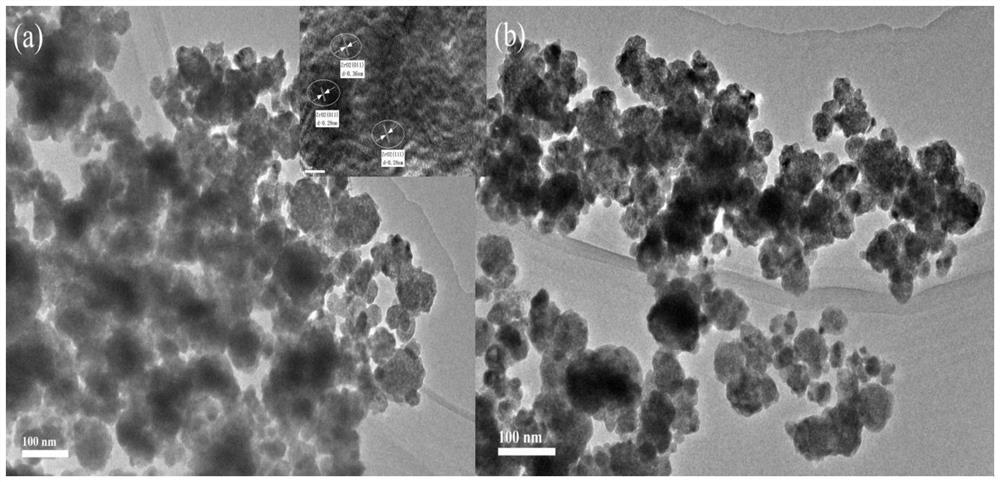
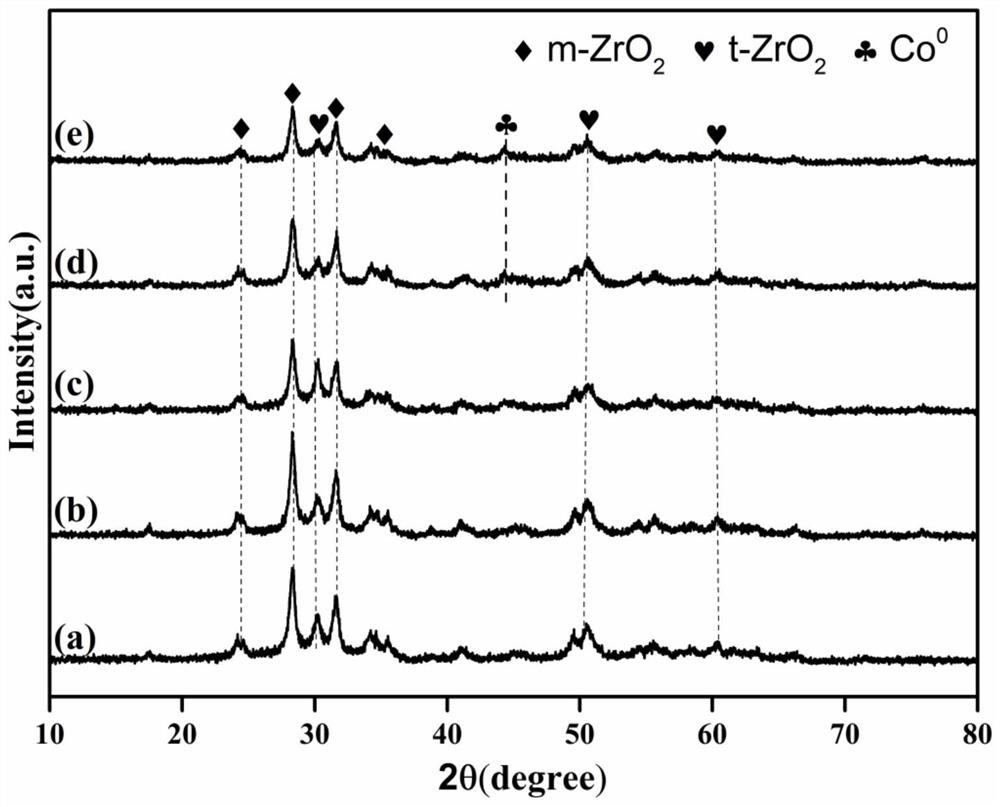
Method for catalytically synthesizing gamma-valerolactone by using cobalt-based hydrogenation catalyst
A hydrogenation catalyst, valerolactone technology, applied in chemical instruments and methods, metal/metal oxide/metal hydroxide catalyst, physical/chemical process catalyst, etc., can solve the problem of poor stability, long reaction time, metal activity The problem of easy leaching of components can achieve the effects of easy recovery, short reaction time and good reusability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] 1. Cobalt-based hydrogenation catalyst Co / ZrO 2 -Nb 2 o 5 preparation of
[0038] The first step: Nb(HC 2 o 4 ) 5 with ZrOCl 2 ·8H 2O adds deionized water and stirs and dissolves in a molar ratio of 0.25:1 to form a mixed solution with a total molar concentration of 0.15mol / L, then slowly adds ammonia water to control the pH value of the mixed solution at 13, and stirs at room temperature for 30min to obtain a Mixed solution containing white precipitate;
[0039] The second step: transfer the above mixed solution containing white precipitate into a polytetrafluoroethylene liner for crystallization at 180°C for 6 hours, cool to room temperature, filter the precipitate with suction, wash the filter cake with deionized water until neutral, and dissolve the obtained The white powdery filter cake was dried at 80°C for 6h, then placed in a box-type muffle furnace at a rate of 2°C / min to 450°C and roasted for 6h, and then ZrO was obtained after cooling. 2 -Nb 2 o 5 ...
Embodiment 2
[0046] Example 2 The operating steps are the same as in Example 1, but the reaction temperature is 160°C, the molar yield of gamma-valerolactone is 13.17%, and the product purity is 20.44%.
Embodiment 3
[0047] Example 3 The operating steps are the same as in Example 1, but the reaction temperature is 180° C., the molar yield of gamma-valerolactone is 86.49%, and the product purity is 92.04%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com