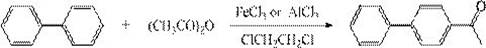Preparation method of 4-hydroxybiphenyl
A technology of hydroxybiphenyl and biphenyl is applied in the field of preparation of 4-hydroxybiphenyl, and can solve the problems of cumbersome post-processing, high preparation price, high cost and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] This embodiment provides a process for synthesizing 4-hydroxybiphenyl, and the specific synthesis steps are as follows:
[0041] Add 1400kg of chloroform to a 2000L enamel reaction kettle, then add 182kg of aluminum trichloride, stir in a cold bath to cool down to -10°C, then add 300kg of biphenyl, continue to cool down to -15°C, and start adding 102kg of acid anhydride dropwise for about 1 hour After dropwise addition, keep warm for 3 hours, add 1500kg of water under stirring, complete hydrolysis, separate liquid, wash with water until neutral, separate clean water, desolventize under normal pressure, keep warm for later use, output 366 kg, content>97.3%, yield 96%.
[0042] In a 3000L kettle, add acylated biphenyl, 1170kg of benzene, lower the temperature to 10°C, add 380kg of 50% hydrogen peroxide dropwise, keep the temperature at 20°C for 9 hours, after the reaction is completed, filter and wash to obtain a crude product, the filtrate is extracted with toluene, Pac...
Embodiment 2
[0044] Add 1350Kg of dichloromethane into a 2000L reactor, then add 405Kg of ferric chloride, stir in a cold bath to cool down to -10°C, then add 300Kg of biphenyl, continue to cool down to -15°C, and start to drop 180Kg of acetyl chloride, about Add dropwise for 1 hour, keep warm for 3 hours, add 1600Kg of water under stirring, complete hydrolysis, separate liquid, wash with water until neutral, separate clean water, precipitate at normal pressure, reach 110°C, after cooling slightly, transfer to stainless steel kettle while hot , output 354Kg, content>98.4%, yield 93%.
[0045]In a 3000L kettle, add acylated biphenyl and 1230kg of acetic acid. After dissolving, cool down to 10°C, add 238kg of peracetic acid dropwise, keep warm at 20°C for 8h, after the reaction is complete, filter and rinse to obtain a crude product, and the filtrate is extracted with toluene Afterwards, it is packed into barrels (for biochemical treatment of sewage), the crude product is dried, distilled un...
Embodiment 3
[0047] Add 1210Kg of dichloroethane into the 2000L reactor, then add 365Kg of zinc chloride, stir in the cold bath to cool down to -10°C, then add 300Kg of biphenyl, continue to cool down to -15°C, and start to drop 110Kg of acid anhydride for about 1 hour After the dropwise addition, keep warm for 4 hours, add 1600Kg of water under stirring, complete the hydrolysis, separate the liquid, wash with water to neutrality, separate the water, and precipitate at normal pressure, to 110 degrees, after a little cooling, transfer it into a stainless steel kettle while it is hot, The output is 288Kg, the content is >98.6%, and the yield is 91%.
[0048] In a 3000L kettle, add acylated biphenyl, 1026kg of dioxane, 623kg of potassium persulfate, drop the temperature to 10°C, add 480kg of 60% sulfuric acid dropwise, keep warm at 20°C for 12h, after the reaction is complete, filter and rinse to obtain The crude product, the filtrate was extracted with toluene, then packed into barrels (for ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com