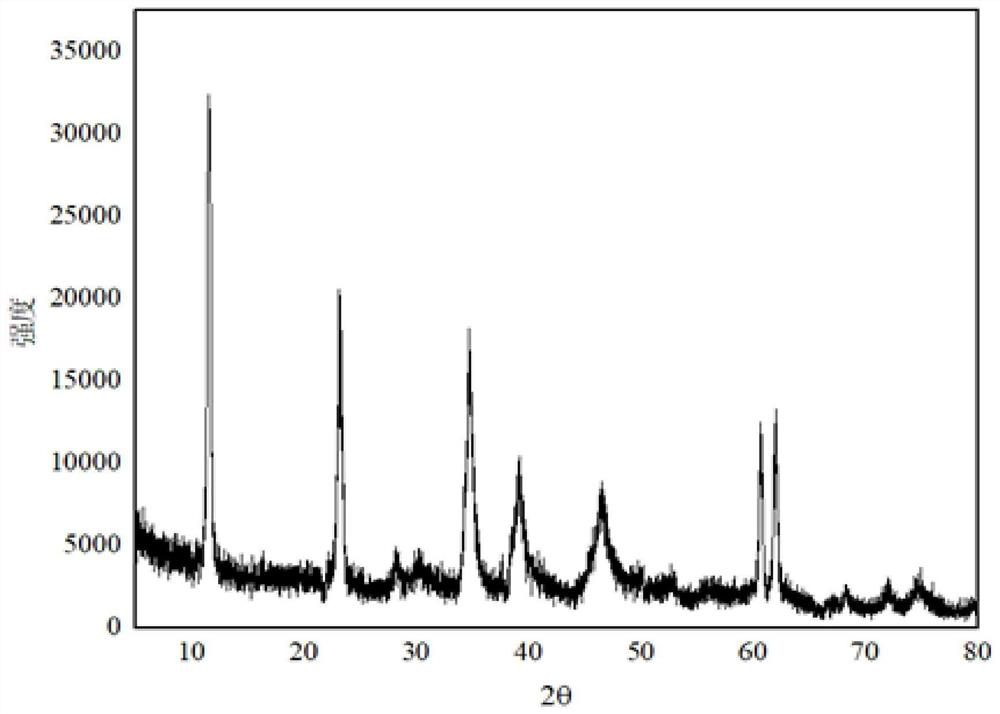
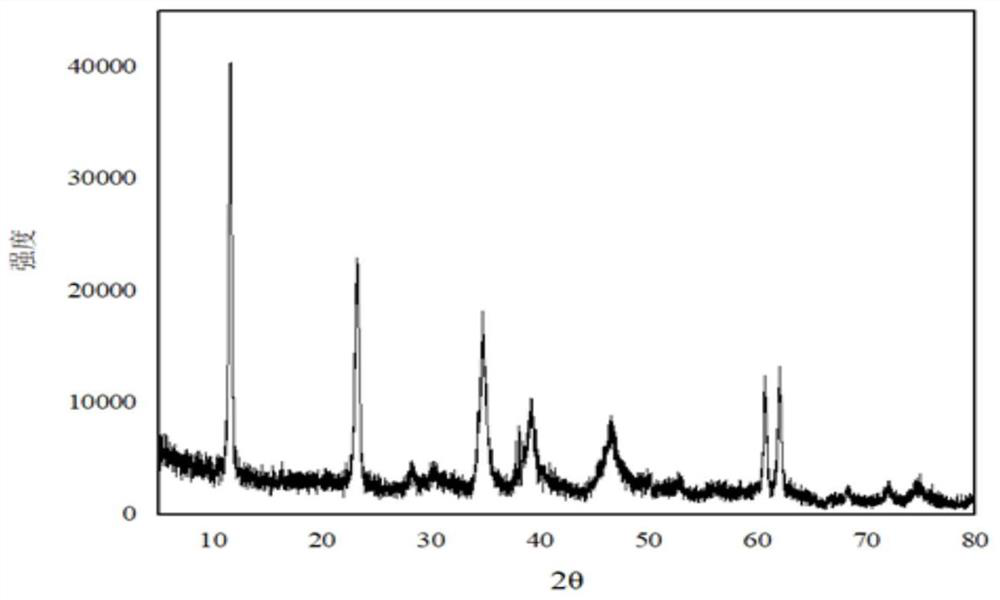
Preparation method for improving thermal stability of magnesium-aluminum hydrotalcite
A technology of magnesium-aluminum hydrotalcite and thermal stability, applied in the directions of hydrotalcite, chemical instruments and methods, aluminum compounds, etc., can solve the problems of high production cost, large degree of agglomeration, low crystallinity of magnesium-aluminum hydrotalcite, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Weigh 29g of magnesium hydroxide (D50=4.0μm), 15.6g of aluminum hydroxide (D50=5.0μm), n(Mg / Al)=2.1, add 145g of water and stir evenly, heat to 65°C, drop into the solution Add 26.25 g of 60% nitric acid and continue stirring for 30 min. Transfer the material to a ball mill, grind at 400r / min for 80min, transfer the ball-milled material to a reaction flask, add dropwise a mixed alkali solution of liquid caustic soda and sodium carbonate until the pH is 12.2, and continue stirring for 30min. The material was transferred to a hydrothermal synthesis kettle for crystallization at 230°C for 24h. After the crystallization is completed, the sample is suction filtered, washed and dried to obtain magnesium aluminum hydrotalcite. The obtained magnesium aluminum hydrotalcite D50=650nm, the 1% dehydration temperature of the sample is 138°C, and the oil absorption of the sample is 40mL / 100g.
Embodiment 2
[0033] Weigh 29g of magnesium hydroxide (D50=1.5μm), 17g of aluminum hydroxide (D50=2μm), n(Mg / Al)=2.9, add 145g of water and stir evenly, heat to 65°C, and dropwise add 60 % nitric acid 26.25g, continue stirring for 30min. Transfer the material to a ball mill, grind for 120min at 400r / min, transfer the ball-milled material to a reaction flask, add dropwise a mixed alkali solution of liquid caustic soda and sodium carbonate until the pH is 11.9, and continue stirring for 30min. The material was transferred to a hydrothermal synthesis kettle for crystallization at 150°C for 16h. After the crystallization is completed, the sample is suction filtered, washed and dried to obtain magnesium aluminum hydrotalcite. The obtained magnesium aluminum hydrotalcite D50=450nm, the 1% dehydration temperature of the sample is 120°C, and the oil absorption of the sample is 75mL / 100g.
Embodiment 3
[0035] Weigh 29g of magnesium hydroxide (D50=3μm), 19.5g of aluminum hydroxide (D50=4μm), n(Mg / Al)=2.3, add 145g of water and stir evenly, heat to 65°C, and dropwise add 60 % nitric acid 26.25g, continue stirring for 30min. Transfer the material to a ball mill, grind at 400r / min for 60min, transfer the ball-milled material to a reaction flask, add dropwise a mixed alkali solution of liquid caustic soda and sodium carbonate until the pH is 11.5, and continue stirring for 30min. The material was transferred to a hydrothermal synthesis kettle for crystallization at 220°C for 20h. After the crystallization is completed, the sample is suction filtered, washed and dried to obtain magnesium aluminum hydrotalcite. The D50 of the magnesium aluminum hydrotalcite obtained through detection is 620nm, the water loss temperature of the sample is 133°C, and the oil absorption of the sample is 53mL / 100g.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| oil absorption | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com