Subunit influenza vaccine split agent and application thereof
A technology of influenza vaccine and subunit, which is applied in antiviral agents, antisense single-stranded RNA viruses, medical preparations containing active ingredients, etc., and can solve problems such as vaccine side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
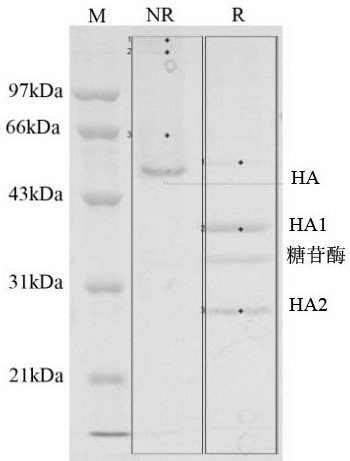
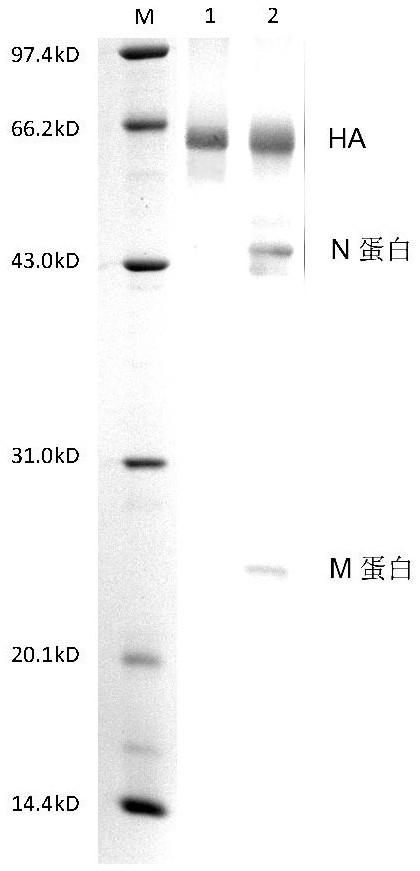
[0034] Preparation of virus particles: take 9-11 day-old chicken embryos and inoculate them with influenza virus virus seeds (the virus type A H1N1 is purchased from NIBSC in the UK, and the virus strains are determined by the annual epidemic strains, according to the seasonal influenza virus distribution experiment stipulated by WHO room), the purchased viruses were thawed in a 37°C water bath, and then inoculated into the allantoic cavity of chicken embryos; cultivated at 35°C for 72 hours, harvested the allantoic fluid, and passed through 0.1m 2 Ultrafiltration membrane bag (product of PALL company, molecular weight cut-off: 100 KDa) was concentrated to one-tenth of the original volume by ultrafiltration, and set aside.
Embodiment 2
[0036] Preparation of virus particles: take 9-11 day-old chicken embryos and inoculate them with influenza virus virus seeds (the virus type B YAMAGATA was purchased from NIBSC in the UK, and the virus strains are determined by the annual epidemic strains, according to the seasonal influenza virus distribution experiment stipulated by WHO room), the purchased viruses were thawed in a 37°C water bath, and then inoculated into the allantoic cavity of chicken embryos; cultivated at 35°C for 72 hours, harvested the allantoic fluid, and passed through 0.1m 2 The ultrafiltration membrane bag (product of PALL company, molecular weight cut-off 1000 KDa) was concentrated to one-eighth of the original volume by ultrafiltration, and was set aside.
Embodiment 3
[0038] Preparation of virus lysate:
[0039] Take 2.9 g of sodium hydroxide and 13 g of sodium dihydrogen phosphate, stir and dissolve in 500 ml of distilled water, then weigh nonoxynol-9 by 1.5 g, stir and dissolve, add 600 g of sucrose, after dissolving, add water to 1000 ml (liquid A);
[0040] Another 2.9 g of sodium hydroxide and 13 g of sodium dihydrogen phosphate were stirred and dissolved in 500 ml of distilled water, then 1.5 g of nonoxynol-9 was weighed, stirred and dissolved, and 100 g of sucrose was added. After dissolving, add water to 1000 ml ml (liquid B);
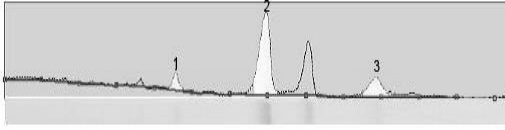
[0041] Add solution A to a zonal centrifuge rotor with a rotational speed of 10000×g and centrifuge for 5 minutes, then add solution B; continue to centrifuge at 25000×g for 45 minutes to form a density gradient and then continuously add 5000 mL of the A / H1N1 virus concentrate prepared in Example 1 After adding the sample, continue to centrifuge at 4°C for 2 h; then add 100 ml of 60% sucrose solution, and ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com