Application of compound as or in preparation of cannabinoid receptor agonist or antagonists
A technology of cannabinoid receptors and agonists, applied in the direction of anti-toxic agents, anti-inflammatory agents, drug combinations, etc., can solve the problems of unreported activity and function
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
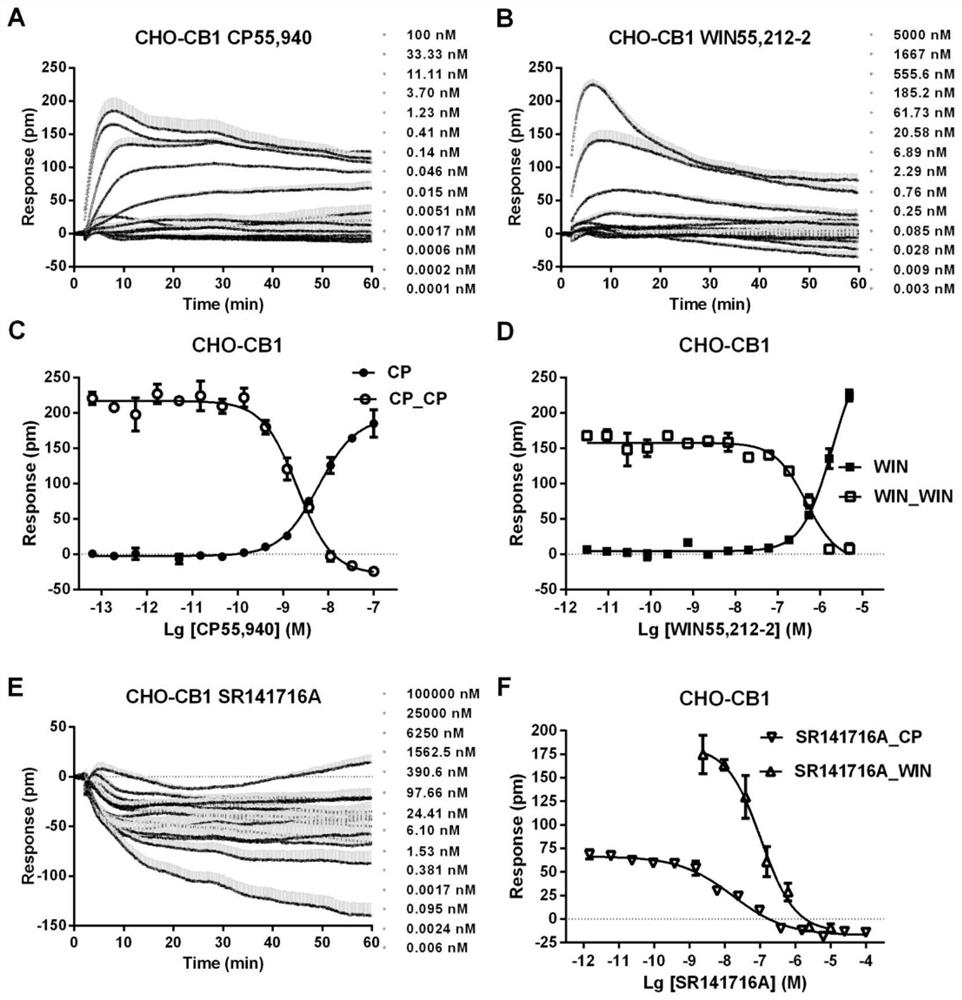
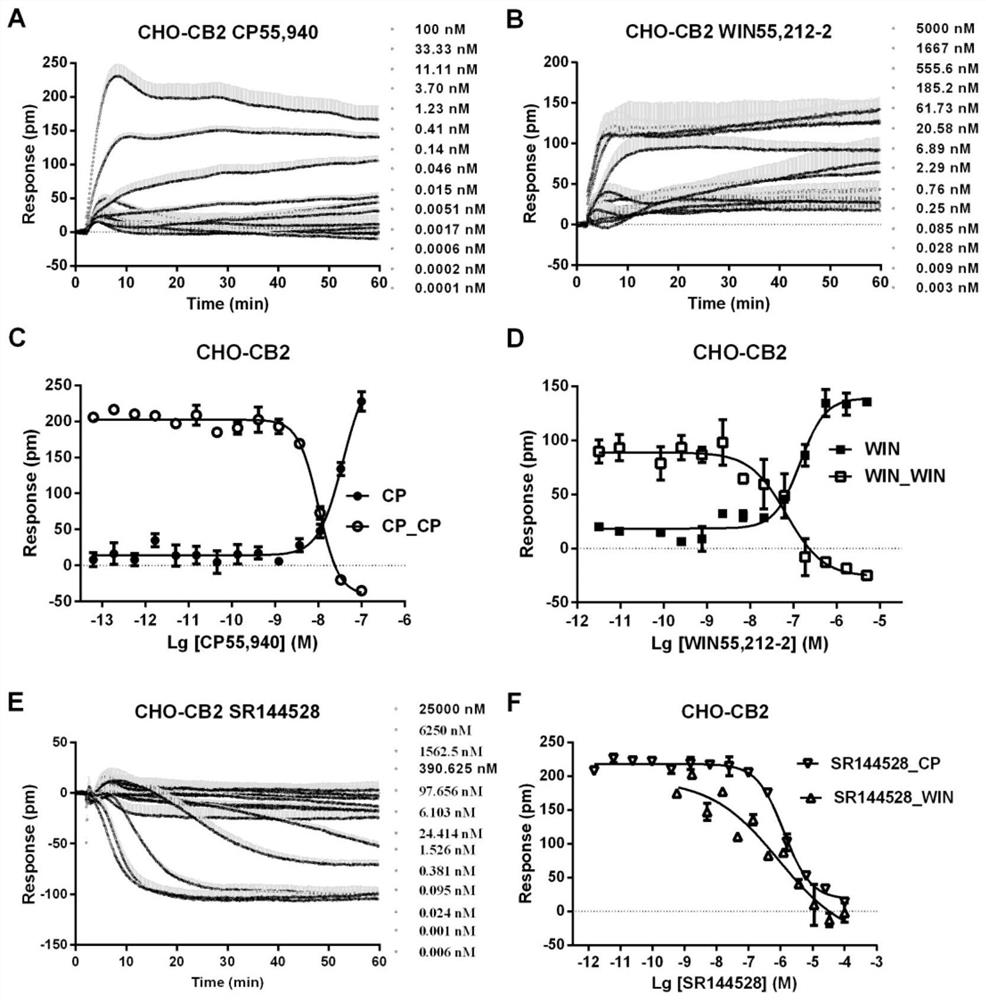
[0050] Example 1: Construction of Cannabinoid Type I Receptor and Cannabinoid Type II Receptor Screening Models
[0051] Materials: Agonist probe CP55,940 was purchased from Sigma-Aldrich, WIN55,212-2 was purchased from AbsinBioscience Inc.; antagonist probes SR141716A and SR144528 were purchased from Tocris Biosciences. Our laboratory stably transfected cannabinoid type I receptor and cannabinoid type II receptor in CHO (Chinese Hamster Ovary, Chinese hamster ovary cells) to obtain CHO-CB1 and CHO-CB2 cell lines [references for transfection process] Holmen, S.L., Vanbrocklin, M.W., Eversole, R.R., Stapleton, S.R., Ginsberg, L.C., 1995. Efficient Lipid-Mediated Transfection of DNA into Primary Rat Hepatocytes. In Vitro CellDev-An 31(5), 347-351.]. The detection platform is the third generation of Corning Imager, the detection signal is the wavelength shift caused by dynamic mass redistribution (Dynamic Mass Redistribution, DMR) of cells.
[0052] The transfected cells CHO-C...
Embodiment 2
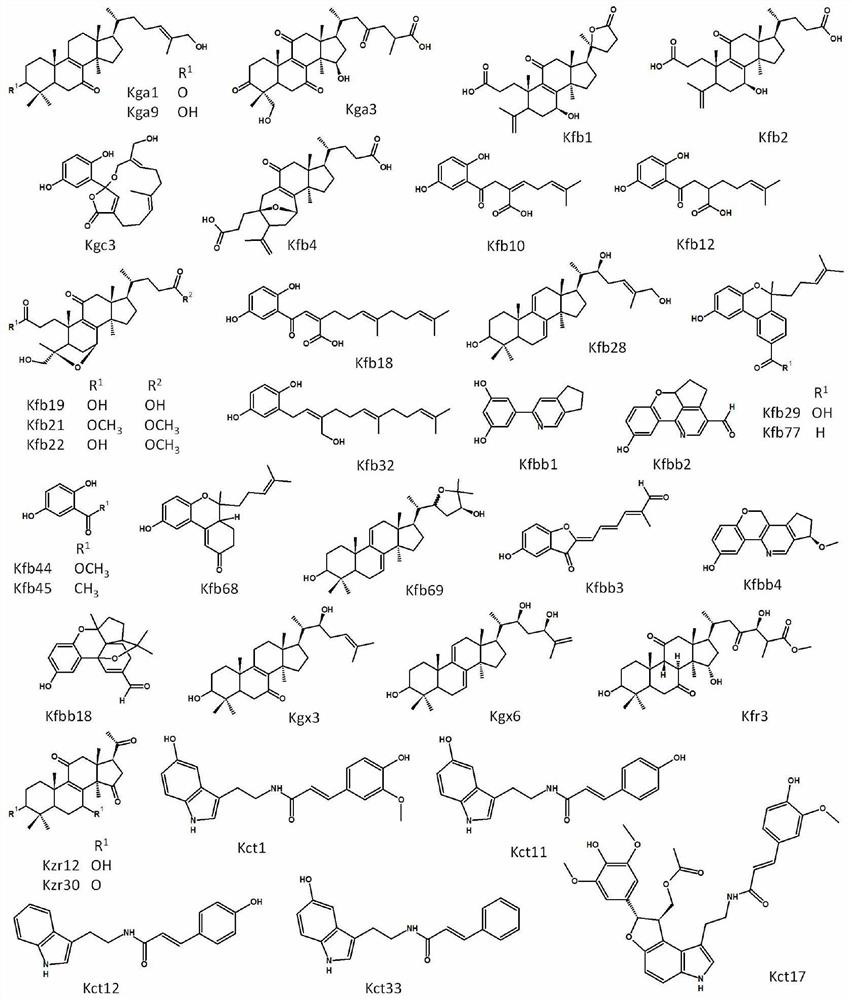
[0064] Example 2: Preliminary Screening of Natural Compounds from Five Plants and Five Fungi on Cannabinoid Receptor Type I Receptor and Cannabinoid Type II Receptor Models
[0065] Materials: 82 natural compounds from five plants and five fungi were provided by Kunming Institute of Botany, Chinese Academy of Sciences. The five plants are safflower seed oil cake (Carthamus tinctorius L), Qingyang ginseng (Cynanchumotophyllum Schneid.), coffee (Coffee Arabica), green thorn fruit (Prinsepia utilis Royle), Maca (Lepidium meyenii, maca); five fungi Ganoderma hainanense, Ganoderma capense, Ganoderma cochlear, Ganoderma resinaceum and Ganoderma applanatum. Natural compound structures see Figure 3-5 [Isolation, purification and characterization of natural compounds related information references:
[0066]Dong, J.R., Peng, X.R., Li, L., Lu, S.Y., Zhou, L., Qiu, M.H., 2018. C21 steroidalglycosides with cytotoxic activities from Cynanchum otophyllum. Bioorganic & medicinal chemistry ...
Embodiment 3
[0093] Example 3: Characterization of Function and Activity of Active Compounds at Cannabinoid Type I and Cannabinoid Type II Receptors
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com