Carbazolo-aromatic ring thermal activation delayed fluorescence material and organic electroluminescent device thereof
A technology of thermally activated delayed and fluorescent materials, which is applied in luminescent materials, electrical solid devices, organic chemistry, etc., can solve the problems of short delay life luminous efficiency, efficiency attenuation, long delay life, etc., to reduce intermolecular aggregation and low efficiency Attenuation, reducing the effect of delay life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
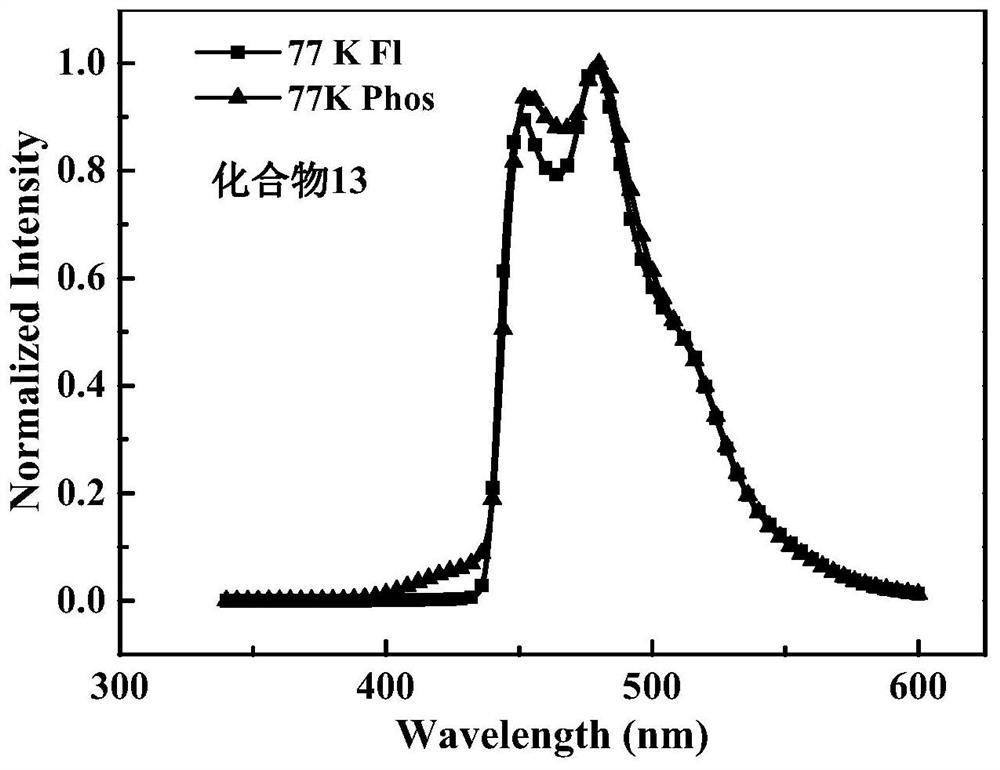
[0056] The heat-activated delayed fluorescent material of carbazolo aromatic ring of the present invention 1312'-(2-(4,6-diphenyl-1,3,5-triazin-2-yl)phenyl)-12'H -Spiro[fluorene-9,7'-indeno[1,2-a]carbazole] can be synthesized by the following method.
[0057]
[0058] (1) In a dry 100ml two-necked flask, 4-bromo-9,9'-spirobis[fluorene] (3.00g, 7.6mmol), 2-chloroaniline (1.07g, 8.4mmol), palladium acetate ( Add 0.34g, 1.5mmol), DPPF (0.83g, 1.5mmol) and sodium tert-butoxide (2.20g, 22.8mmol) in sequence, then add 40mL of dry toluene, stir rapidly and blow a large amount of nitrogen for 5-10 minutes, under the protection of nitrogen Stir at reflux for 5 hours. Cooling, extraction first, spin-drying, column chromatography with petroleum ether and dichloromethane can give intermediate 1-1(N-(2-chlorophenyl)-9,9'-spirobis[fluorene]-4-amine ), yield 63%.
[0059] (2) Add intermediate 1-1 (3.00g, 6.7mmol), palladium acetate (0.30g, 1.3mmol), potassium carbonate (4.62g, 33.5mmol...
Embodiment 2
[0063] The carbazolo aromatic ring thermally activated delayed fluorescent material prepared in Example 1 has the formula 13(12'-(2-(4,6-diphenyl-1,3,5-triazin-2-yl)phenyl )-12'H-spiro[fluorene-9,7'-indeno[1,2-a]carbazole]) as light-emitting guest to prepare devices.
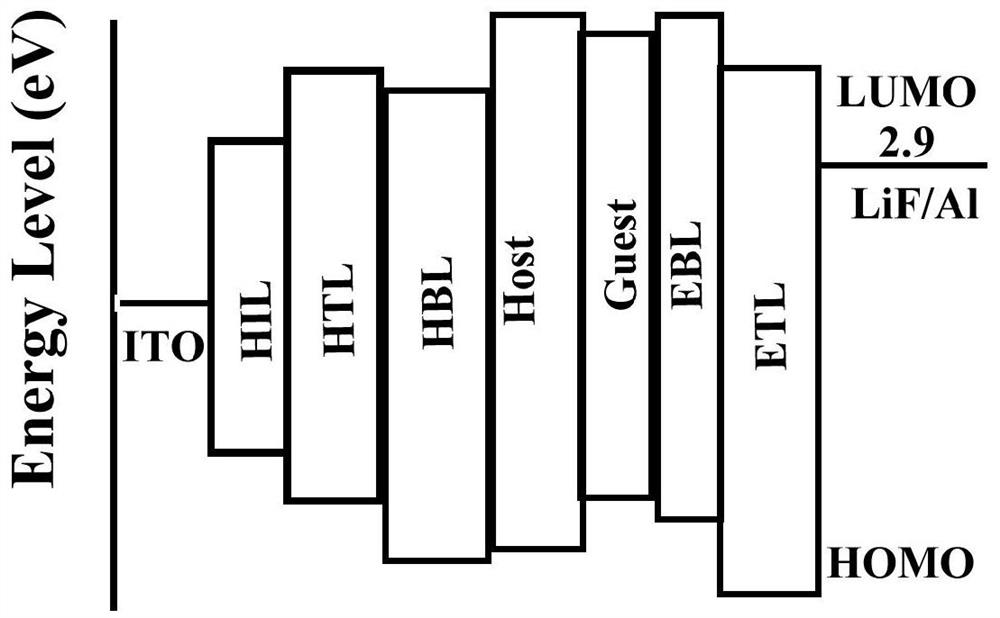
[0064] This example demonstrates the performance verification of an organic electroluminescent device prepared with 13 as a guest luminescent material. The ITO (Indium Tin Oxide) glass was ultrasonically cleaned in detergent and deionized water for 30 minutes sequentially. Then vacuum dry for 2 hours (105°C), then put the ITO glass into the plasma reactor for 5 minutes of oxygen plasma treatment, transfer it to the vacuum chamber to prepare organic film and metal electrode, and then prepare a layer of 10nm by vacuum evaporation. The hole injection material molybdenum trioxide, followed by evaporation of 60nm thick hole transport material: 4,4'-bis[N-(1-naphthyl)-N-phenylamino] biphenyl (NPB), and then evaporati...
Embodiment 3
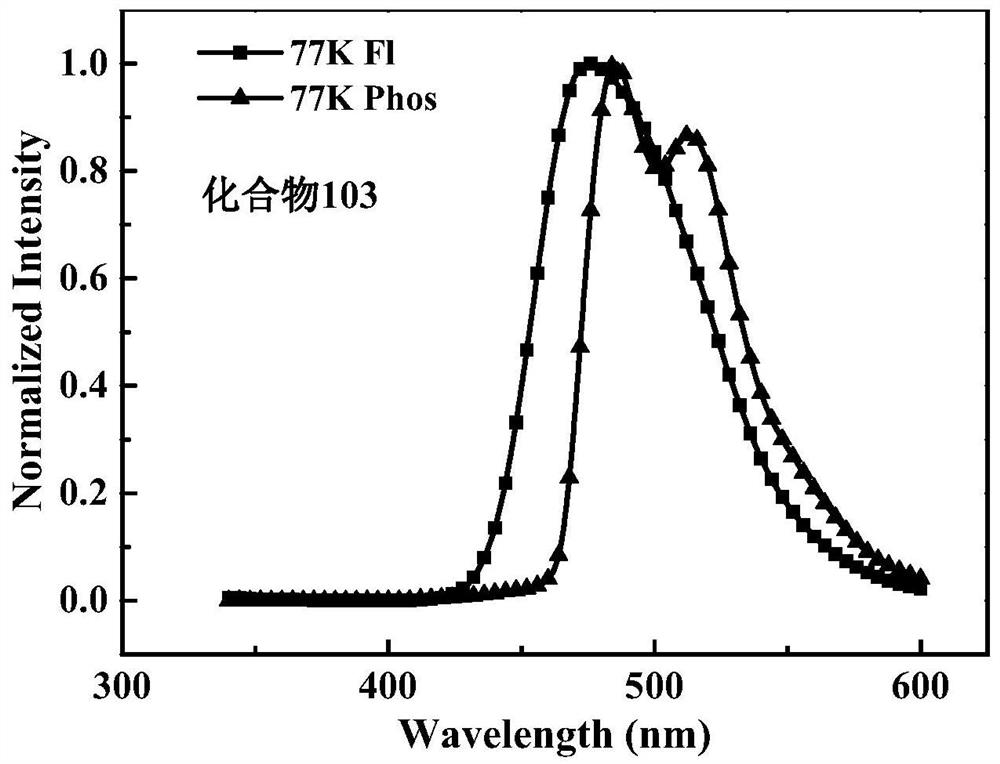
[0067] The 23(7,7-dimethyl-12-(3-(pyrimidin-2-yl)pyridin-2-yl)-7,12-dihydroindeno[1,2-a]carba Azole) can be synthesized by the following method.
[0068]
[0069] (4) In a dry 100ml two-necked flask, mix 4-bromo-9,9-dimethylfluorene (2.07g, 7.6mmol), 2-chloroaniline (1.07g, 8.4mmol), palladium acetate (0.34g , 1.5mmol), DPPF (0.83g, 1.5mmol) and sodium tert-butoxide (2.20g, 22.8mmol) were added sequentially, and then 40mL of dry toluene was added, and a large amount of nitrogen gas was stirred rapidly for 5-10 minutes, and reflux was stirred under nitrogen protection. 5 hours. After cooling, extract first, spin dry, and use petroleum ether and dichloromethane column chromatography to obtain intermediate 3-1 with a yield of 70%.
[0070] (5) Add intermediate 3-1 (2.14g, 6.7mmol), palladium acetate (0.30g, 1.3mmol), potassium carbonate (4.62g, 33.5mmol), tricyclohexylphosphine fluoroboron to a dry 100ml three-necked flask salt (1.43g, 3.9mmol), take 20ml of N,N-dimethylace...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com