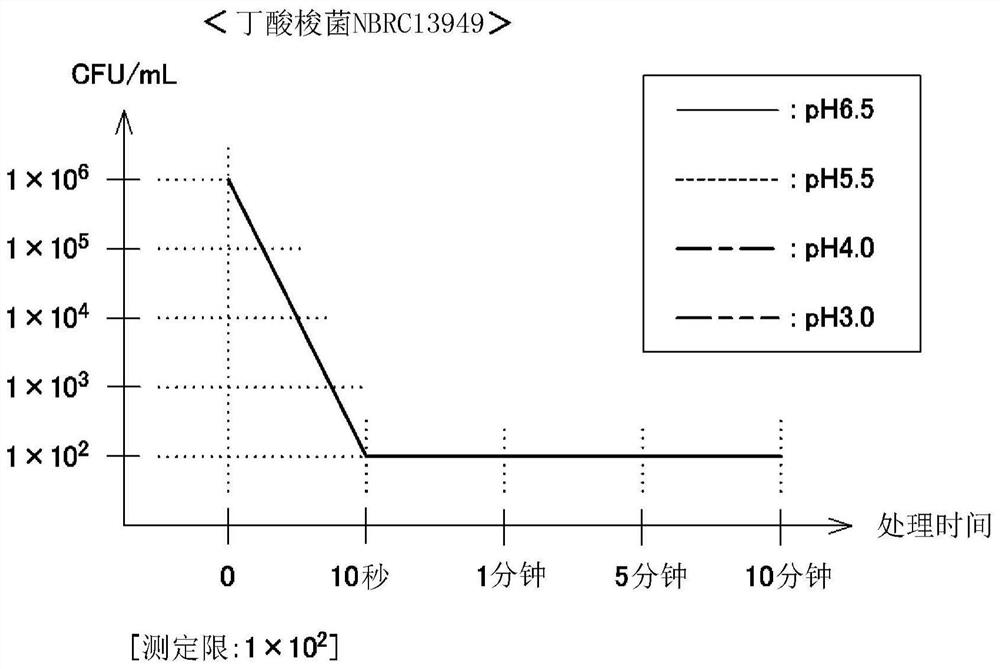
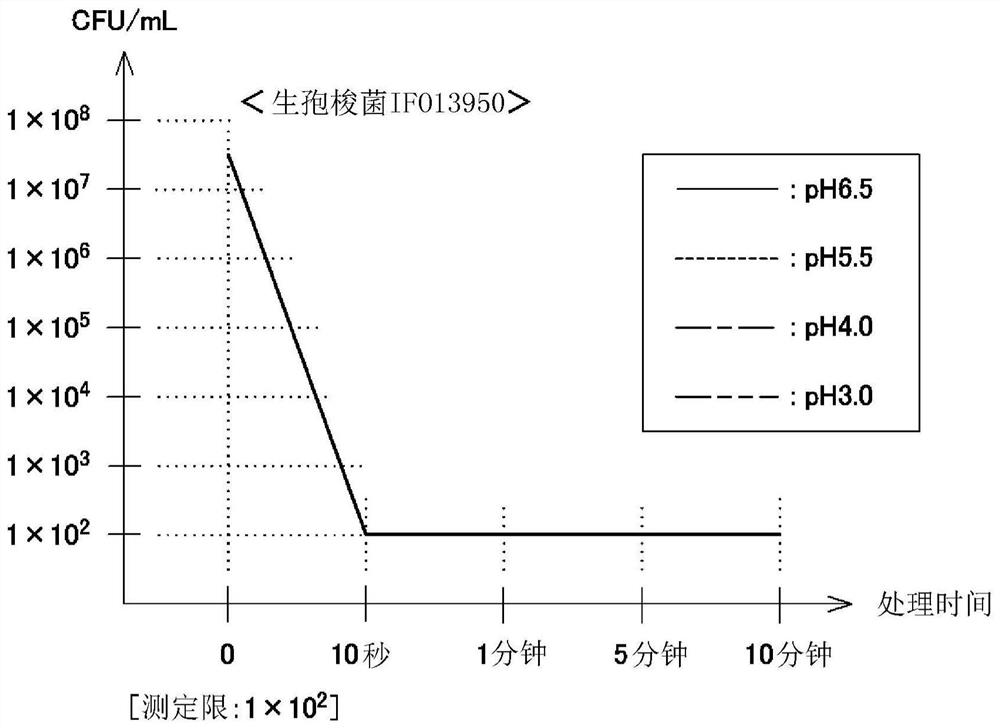
Antimicrobial agent containing hypochlorous acid
An antimicrobial and microorganism technology, applied in the directions of biocides, biocides, antifungals, etc., can solve problems such as danger and chlorine generation, and achieve excellent bactericidal effect and safety effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] (Aqueous solution of hypochlorous acid and its production example)
[0051] Weigh sodium hypochlorite according to 0.026% (W / V), mix with purified water for dilution. Diluted hydrochloric acid (approximately 9.5 to 10.5% (W / V)) was added to the sodium hypochlorite aqueous solution prepared by this dilution, and pH was adjusted to 6.0 to 6.5. The residual chlorine concentration in this solution was 220 ppm.
[0052] (Component analysis of hypochlorous acid aqueous solution)
[0053] The component analysis table of the hypochlorous acid aqueous solution is shown below.
[0054] [Table 1]
[0055]
[0056]
[0057] In addition, pure water having the following properties was used in this example.
[0058] [Table 2]
[0059] project properties and characteristics character Colorless, transparent, odorless liquid purity Organic carbon content≤0.50mg / L Conductivity Conductivity (25℃)≤2.1μS / cm
[0060] *Conductivity test: Measure...
Embodiment 2
[0062] (Measurement of antiviral effect on norovirus)
[0063] (1) Test strain
[0064] Norovirus (Norovirus belonging to NV gene group 2) from feces was used. The virus was qualitatively confirmed according to the norovirus detection method (PCR method) recommended by the Japanese Ministry of Health, Labor and Welfare.
[0065] (2) Preparation of the sample to be tested
[0066] The hypochlorous acid aqueous solution was adjusted so that the residual chlorine concentration was 200 ppm, and it was used as a test sample. The following samples were prepared using the above-mentioned test samples for the measurement of the antiviral effect.
[0067] (a) Negative control (500 μL of norovirus suspension)
[0068] (b) Positive control (5-fold dilution: 100 μL of norovirus suspension + 400 μL of purified water)
[0069] (c) Sample (100 μL of norovirus suspension + 400 μL of the tested sample)
[0070] (3) Measurement method
[0071] The proliferation of norovirus was confirmed...
Embodiment 3
[0077] (Determination of the bactericidal effect on bacteria)
[0078] (1) For test sample
[0079] The aqueous hypochlorous acid solution prepared in Example 1 was used. The residual chlorine concentration of the stock solution was 200 ppm. This stock solution was serially diluted 2-fold and diluted to 100 ppm, 50 ppm, 25 ppm, 12.5 ppm and 6.3 ppm for use.
[0080] (2) Test method
[0081] 1) Test bacteria
[0082] Seven kinds of bacteria shown in Table 4 below were used. The stock solution with a residual chlorine concentration of 200ppm was serially diluted 2-fold with sterilized distilled water to prepare dilutions of 100ppm, 50ppm, 25ppm, 12.5ppm, and 6.3ppm, and dispensed 5mL each into a 20mL test tube for use. The concentration of residual chlorine in the sample was measured using a hand-held water quality detector AQ-101 (manufactured by Shibata Scientific Co., Ltd.).
[0083] [Table 4] The type of bacteria to be tested and the minimum bactericidal concentration ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com