Metalloporphyrin complex and preparation method and application thereof
A technology of metalloporphyrins and complexes, which is applied in the direction of pharmaceutical formulations, medical preparations containing active ingredients, antibacterial drugs, etc., can solve the problems of easy drug resistance of drugs, and achieve the reduction of bacterial drug resistance and good antibacterial properties active, good inhibitory effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
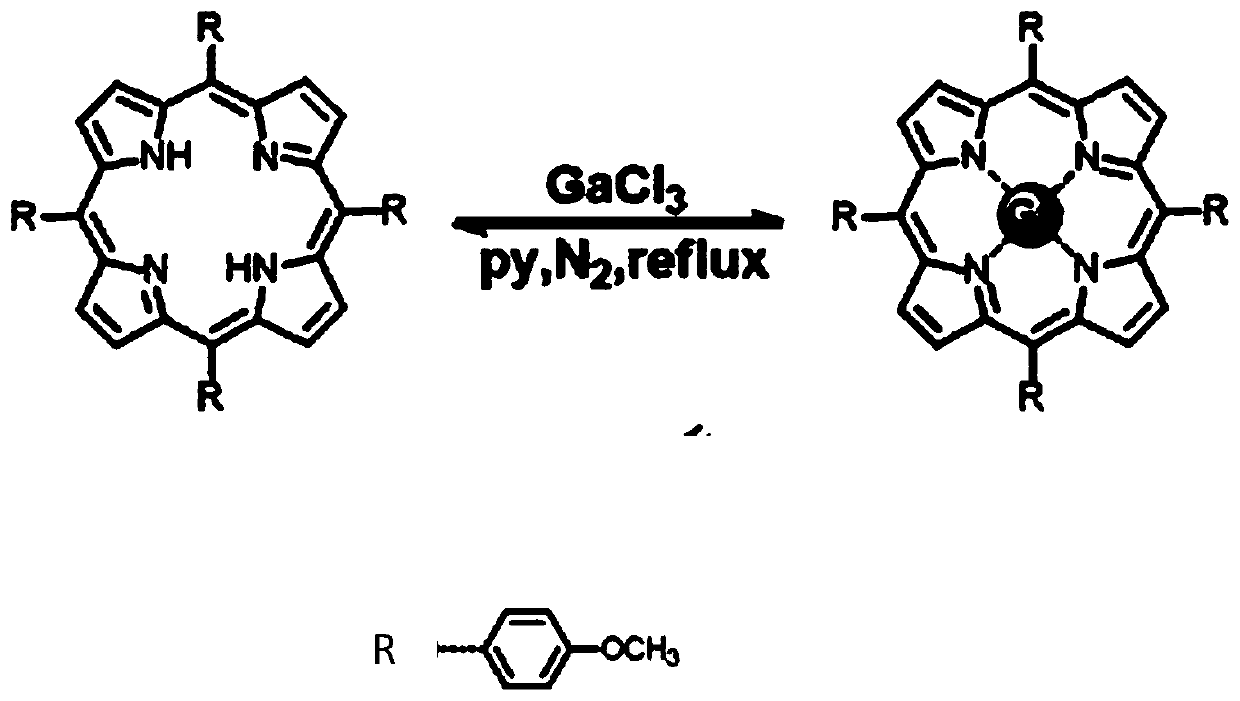
[0027] The synthetic route of embodiment 1 gallium porphyrin complex
[0028] For the synthesis of complexes, weigh a certain amount of porphyrin (1.0mmol) and gallium chloride (0.5mmol) into a 100mL round-bottomed flask, add 30mL of anhydrous pyridine solvent, and heat under reflux under nitrogen protection. Reaction 8h. After the reaction was completed, cool to room temperature to obtain a deep red solution, spin dry to collect the precipitate, and wash with a small amount of pre-cooled ethanol, then wash the crude product with chloroform, filter and dry overnight in a vacuum desiccator to obtain a dark purple powder. The synthesis route of gallium porphyrin complex is as follows figure 1 shown. The NMR data are as follows:
[0029] 1 H NMR (400MHz, DMSO-d 6 )δ9.07(s, 4H), 8.41(m, 3H), 7.63(d, J=0.8Hz, 1H), 8.14(d, J=4.0Hz, 4H), 7.90(t, 7H), 7.43( d, J = 4.0 Hz, 4H), 4.07 (s, 7H).
Embodiment 2
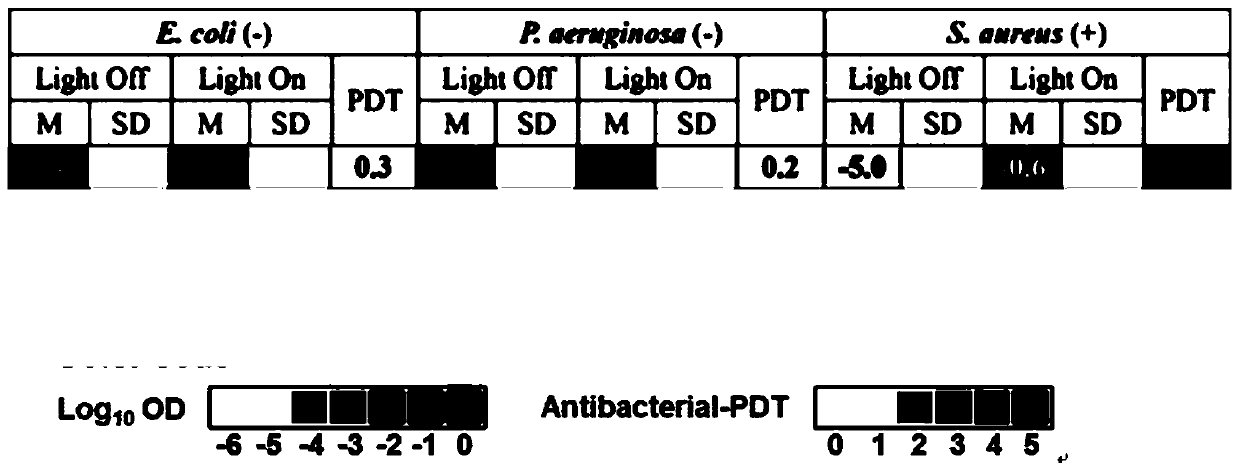
[0030] Example 2 Gallium porphyrin complex antibacterial activity screening
[0031] Gallium porphyrin complexes against bacteria 50 For the determination of the value, dilute the overnight cultured Escherichia coli, Pseudomonas aeruginosa, and Staphylococcus aureus to the logarithmic phase, and then dilute the bacteria to an OD of 0.05 for later use. Take 6 sterile 96-well plates, and each bacteria 2 panels each, one each under light and dark conditions. The gallium porphyrin complex was diluted with the culture medium, and then the two-fold method was used to dilute different drug concentrations (512, 256, 128, 64, 32, 16, 8, 4, 2, 1, 0.5, 0.25 μg / mL) . Add 50 μL of gallium porphyrin complex to each well, and then add 50 μL of diluted bacterial solution, and culture in a 37° C. incubator for 20 h. The culture medium is used as a negative control, and the bacterial solution (without drugs) is used as a positive control. Measure the absorption of OD600 with a microplate rea...
Embodiment 3
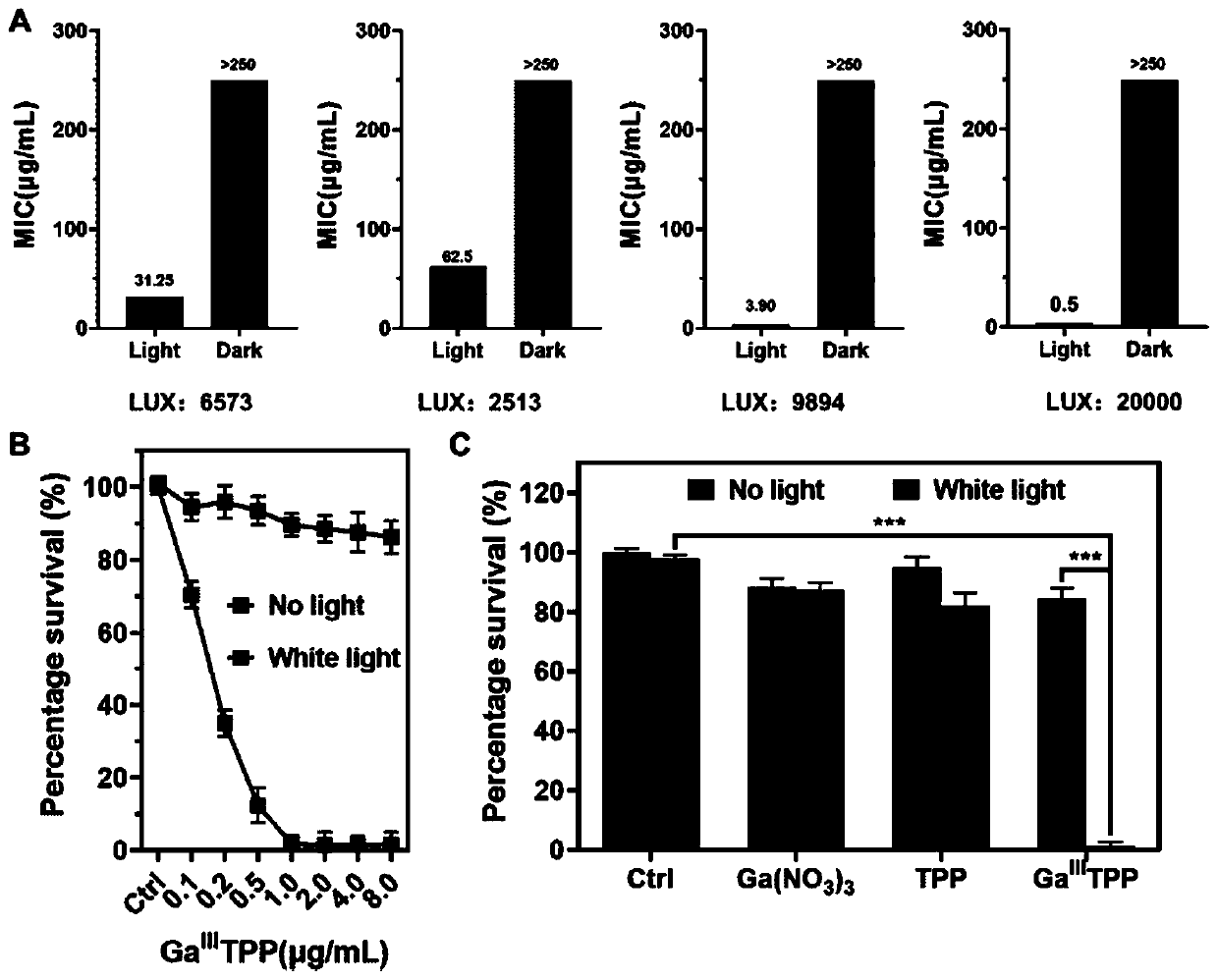
[0032] Example 3 Gallium porphyrin complex antibacterial activity condition optimization
[0033] The Staphylococcus aureus cultured overnight was diluted to the logarithmic phase, and the bacteria were diluted to an OD of 0.05 for use. Take 5 sterile 96-well plates, dilute the gallium porphyrin complex with culture medium, and dilute different drug concentrations (512, 256, 128, 64, 32, 16, 8, 4, 2 , 1, 0.5, 0.25 μg / mL). Add 50 μL gallium porphyrin complex to each well and then add 50 μL diluted bacterial solution. 1 block under dark conditions, and the remaining 4 blocks were irradiated with light intensity of 2513, 6573, 9894, and 20000LUX respectively, and placed in a 37°C incubator for 20h. The culture medium was used as a negative control. object) served as a positive control. The OD600 absorption was measured with a microplate reader, and the MIC value under each light intensity was recorded. image 3 As shown in A, when the light intensity is 20000LUX, the minimum ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com