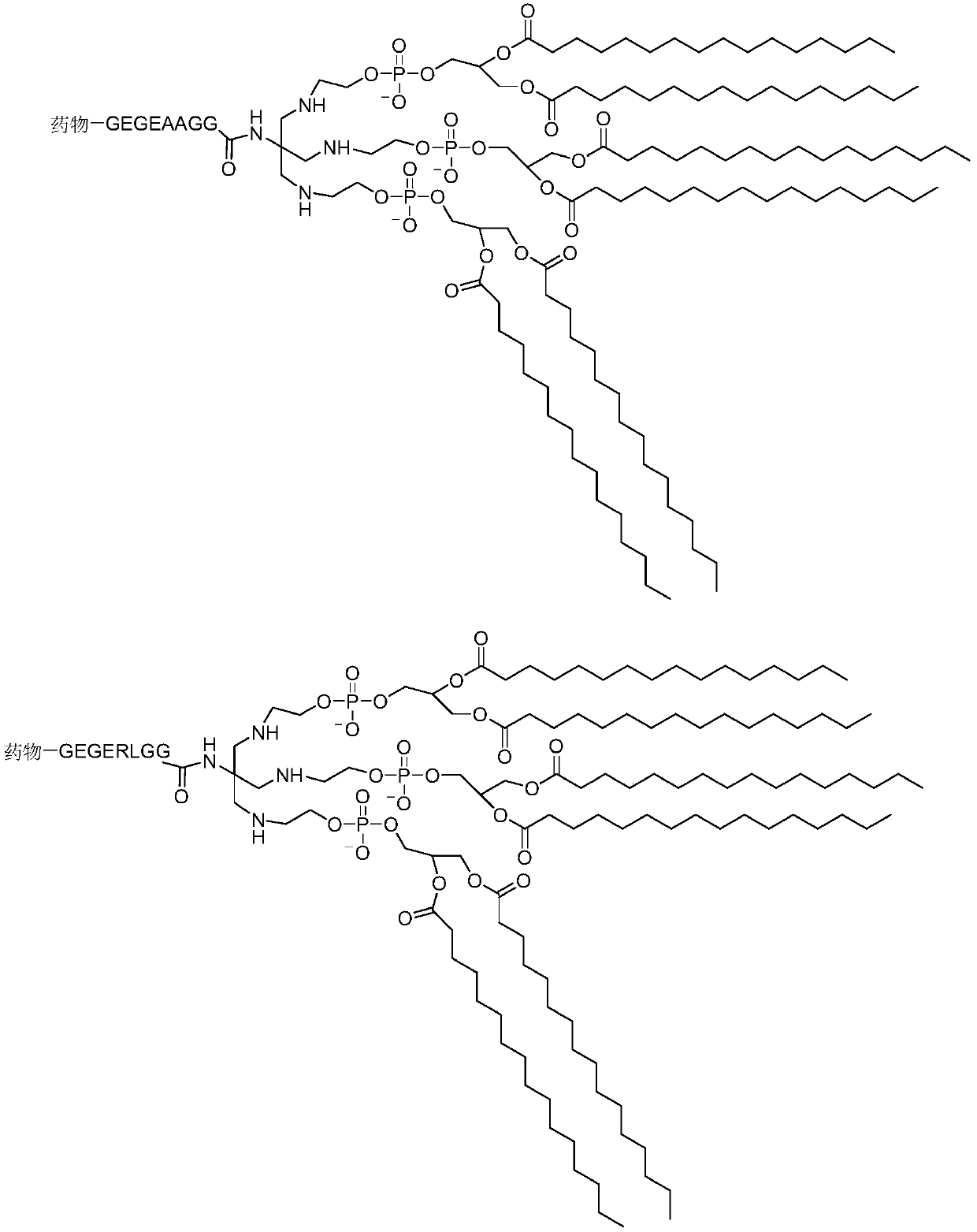
Novel lung intelligent drug release system
A pulmonary and intelligent technology, applied in the field of drug release systems, can solve problems such as insufficient research, and achieve the effect of ensuring intelligent selectivity and improving bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Example 1: Phosphite Synthesis
[0035] Add anhydrous tetrahydrofuran (THF) under dry and nitrogen protection, cool to minus 78 degrees with dry ice, slowly add 78.5 grams of phosphorus trichloride (PCl 3 ) and 67.4 grams of 2,6-lutidine (2,6-lutidine), then 50 grams of 2-azide-ethanol was added dropwise, and the reaction mixture was stirred for 2 hours, during which the temperature rose from minus 78 degrees to minus 30 Spend. Recool the reaction mixture to minus 78 degrees, slowly add 67.4 grams of 2,6-lutidine (2,6-lutidine), then dropwise add 326 grams of diacylglycerol, and stir the reaction mixture for 5 hours, during which The temperature rose from minus 78 degrees to minus 20 degrees. Recool the reaction mixture to minus 78 degrees, slowly add 70 grams of 2,6-lutidine (2,6-lutidine), then slowly add water dropwise, and stir the reaction mixture for 1.5 hours, during which the temperature rises from minus 78 degrees to To minus 30 degrees, the reaction process...
Embodiment 2
[0036] Example 2: Phosphite Oxidation
[0037] The product of 100 grams of embodiment 1 is dissolved in 300 milliliters of toluene (toluene), is cooled to minus 10 degrees, then slowly adds dropwise 28 grams of sulfuryl chloride (SO 2 Cl 2 ), temperature remains no more than 0 degree, stirs 1 hour, removes excess sulfonyl chloride and solvent under reduced pressure distillation at low temperature, then adds 300 milliliters of tetrahydrofuran (THF) and dissolves and cools to zero degree, slowly adds 75 milliliters of water, stirs 3 Hours, the reaction process was monitored by TLC. After the reaction, the excess solvent was distilled off under reduced pressure, and the remaining solvent was dissolved in 600 ml of ethyl acetate, washed with saturated sodium chloride solution, dried over anhydrous sodium sulfate and concentrated to obtain 97 g of product.
Embodiment 3
[0038] Example 3: Reduction of azide group to amine
[0039] Dissolve 90 grams of the product of Example 2 in 300 milliliters of tetrahydrofuran (THF), add 98 grams of triphenylphosphine, raise the temperature to 50 degrees and keep for 3 hours, add dilute hydrochloric acid when the temperature drops to room temperature and stir for 2 hours, the reaction process uses TLC for monitoring. After the reaction, 600 ml of ethyl acetate was added, washed with saturated sodium bicarbonate solution and saturated sodium chloride solution, dried over anhydrous sodium sulfate and concentrated, then purified by column chromatography to obtain 79 g of product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com