Zwitterionic peptides and their derivatives and nanomedicine based on them
A technology of zwitterions and nano-drugs, which is applied in drug combinations, peptide preparation methods, peptides, etc., can solve the problems of unclear biological functions of the hydrophobic inner core of albumin molecules, interference with small-molecule drug loading capacity, etc., and achieve excellent blood phase Capacitance, excellent conversion ability, accelerated release effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
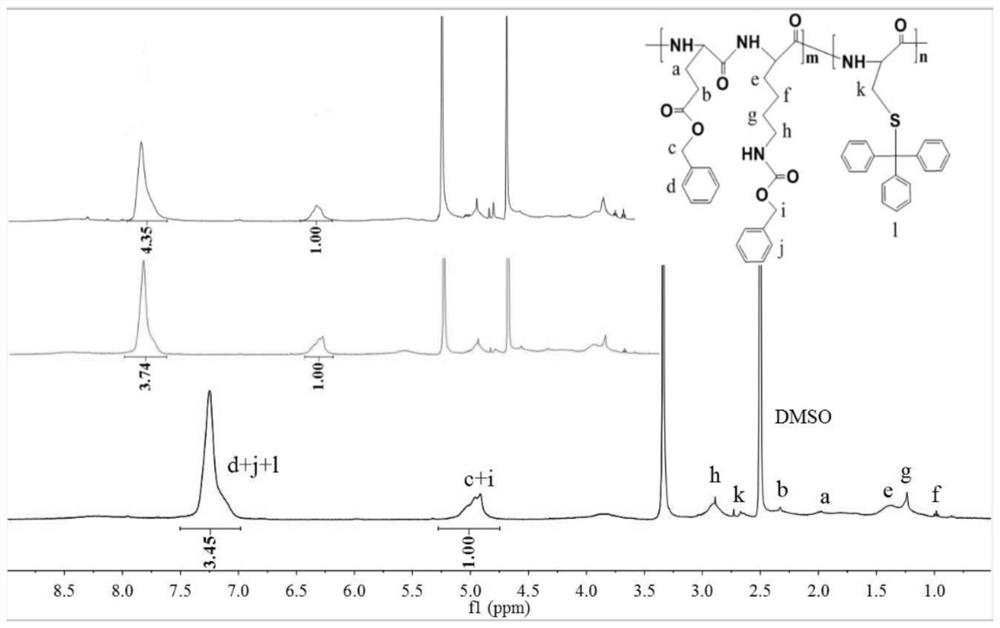
[0090] Example 1: Preparation of doxorubicin-loaded nanomedicine based on glutamic acid lysine dimer (EK) and cysteine (C) (EK-C)
[0091] (1) Synthesis of thiol-activated EK-C polypeptide backbone
[0092] Referring to the synthetic route (1), weigh the EK dimer (2mmol) protected by 1g side chain benzyloxycarbonyl (Z) and the cysteine (H-Cys(Trt) protected by 0.2422g mercaptotrityl group respectively). -OH) (0.667 mmol) was added to a 50 mL round bottom flask, followed by 4 mL of anhydrous DMF solution. Then weigh 0.69g 1-ethyl-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDC·HCl) (3.6mmol), 0.4865g anhydrous N-hydroxybenzotriazole (HOBt ) (3.6mmol) and 0.9306g N,N-diisopropylethylamine (DIEA) solution (7.2mmol) were added to the above-mentioned round bottom flask, sonicated until completely dissolved, then sealed with a parafilm and stirred for 48h. By changing the amount of H-Cys(Trt)-OH, the mixed polypeptide products with EK and C molar ratios of 2:1, 3:1 and...
Embodiment 2
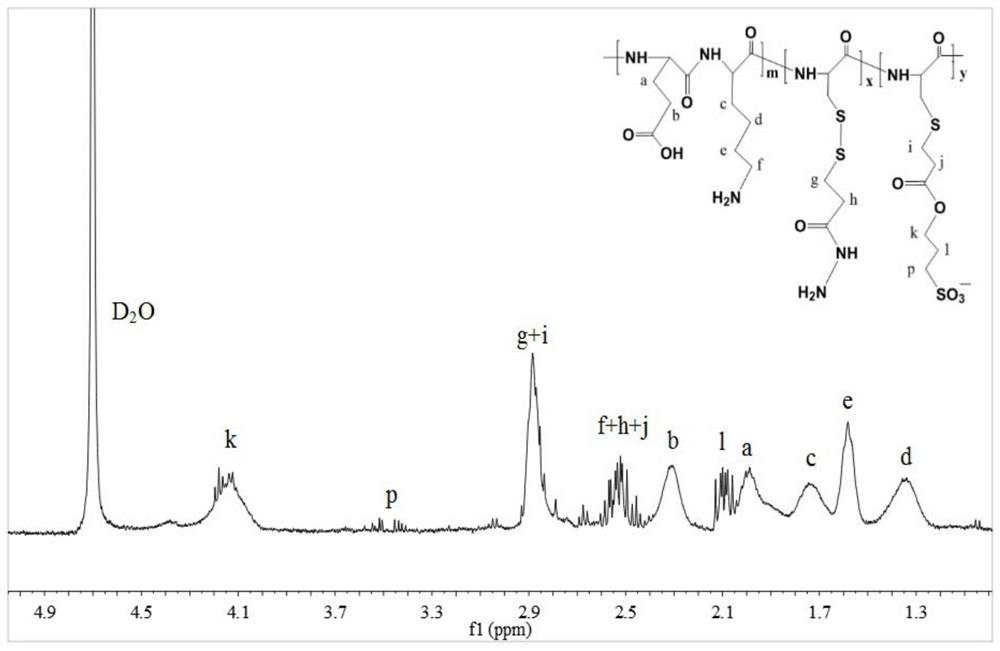
[0106] Example 2: Preparation of doxorubicin-loaded nanomedicine based on glutamic acid lysine dimer (EK), cysteine (C) and aspartic acid (EK-C-D)
[0107] (1) Synthesis of thiol-activated EK-C-D polypeptide backbone
[0108] Refer to the synthetic route (4), weigh 1g side chain Z-protected EK dimer (2mmol), 0.3mmol side chain Z-protected aspartic acid and 0.1817g H-Cys(Trt)-OH (0.5mmol) and add into a 50 mL round bottom flask, then add 4 mL of anhydrous DMF solution. Then weigh 0.69g EDC·HCl (3.6mmol), 0.4865g HOBt (3.6mmol) and 0.9306g DIEA solution (7.2mmol) into the above-mentioned round-bottomed flask, sonicate until completely dissolved, then seal and stir the reaction with a parafilm 48h. By changing the amount of H-Cys(Trt)-OH, the hybrid polypeptide products with EK and C molar ratios of 2:1, 3:1 and 4:1 were synthesized.
[0109] After the reaction, add 10 times the amount of DMF in anhydrous ether to the flask to precipitate the reaction product, and stir for a...
Embodiment 3
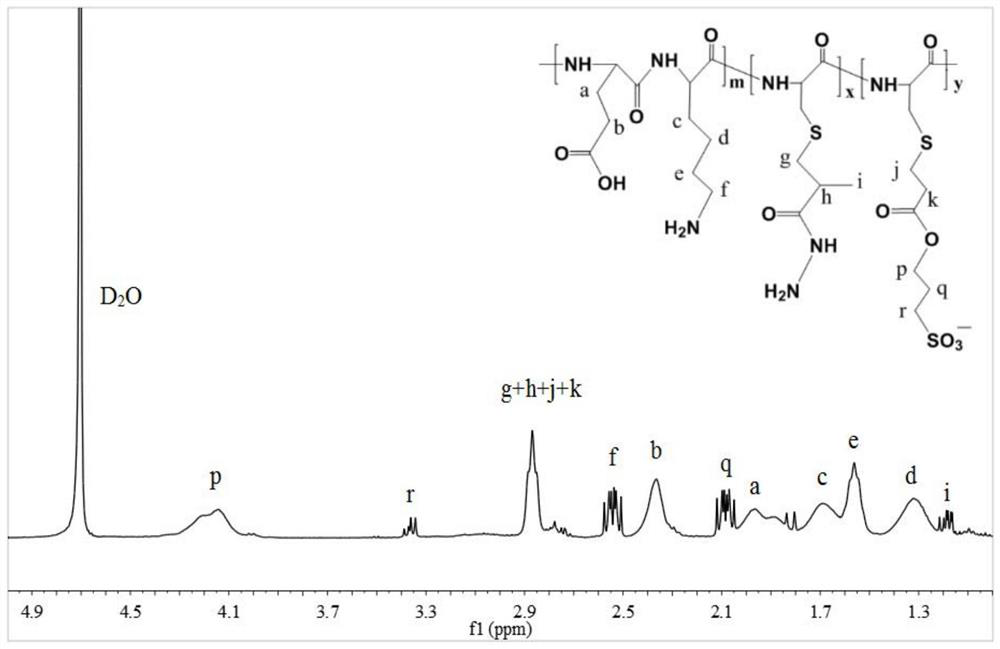
[0118] Embodiment 3: Carrying doxorubicin nanoparticles based on glutamic acid lysine dimer (EK), cysteine (C), aspartic acid and phenylalanine (EK-C-D-F) drug preparation
[0119] (1) Synthesis of EK-C-D-F polypeptide backbone
[0120] Referring to the synthetic route (6), weigh 1g side chain Z-protected EK dimer (2mmol), 0.3mmol side chain Z-protected aspartic acid, 0.1817g H-Cys(Trt)-OH (0.5mmol) and 0.3mmol of phenylalanine (F) was added to a 50mL round bottom flask, and then 4mL of anhydrous DMF solution was added. Then weigh 0.9g EDC·HCl (4.7mmol), 0.64g HOBt (4.7mmol) and 1.21g DIEA solution (9.4mmol) into the above-mentioned round-bottomed flask, sonicate until completely dissolved, then seal with a parafilm and stir for 48h . By changing the addition amount of H-Cys(Trt)-OH, the molar ratio of synthetic EK and cysteine (C) is 2:1, 4:1 and 6:1 mixed polypeptide products respectively; The molar ratios of EK and phenylalanine were 2:1, 3:1 and 4:1 to synthesize t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| degree of grafting | aaaaa | aaaaa |
| degree of grafting | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com