Targeted phase-change nano drug system and its preparation method and application
A nano-drug, targeting technology, applied in the field of biomedicine, can solve problems such as toxicity and side effect targeting, and achieve the effect of improving enzyme activity and reducing the level of reactive oxygen species in podocytes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] Example 1: Preparation of targeted phase-change nano-medicine system (DEX / PFP@LIPs-BMS-α)
[0065] (1) Synthesis of DSPE-PEG-COOH—BMS-α
[0066] Using distearoylphosphatidylethanolamine-polyethylene glycol-carboxyl (DSPE-PEG(2000)-COOH) and compound BMS-470539 (abbreviated as BMS-α) as raw materials, the condensed Compounds (distearoylphosphatidylethanolamine-polyethylene glycol-carboxy-BMS-α, DSPE-PEG-COOH-BMS-α).
[0067] Take 80mg (0.027mmol) of DSPE-PEG(2000)-COOH, dissolve it in 20ml of dimethylformamide (DMF); add 3.8mg (0.027mmol) of anhydrous 1-hydroxybenzotriazole (HOBT); Add 3.5mg (0.027mmol) N, N-diisopropylcarbodiimide (DIC), shake for 0.5h; then add 15mg (0.025mmol) compound BMS-470539 dissolved in 2ml DMF, shake at room temperature for 24h; The target product DSPE-PEG-COOH—BMS-α was obtained through purification. The final target product was detected by Mass Spectrometry and HPLC, and the target product was determined to be DSPE-PEG-COOH—BMS-α.
[0068...
Embodiment 2
[0075] Example 2: Preparation of targeted phase-change nano-medicine system (PFP@LIPs-BMS-α)
[0076] The preparation method of the present embodiment is the same as embodiment 1, and the difference is in step (2), specifically as follows:
[0077] (2) Preparation of lipid film
[0078] Dipalmitoylphosphatidylcholine (DPPC), dipalmitoylphosphatidylglycerol (DPPG), the compound (DSPE-PEG-COOH-BMS-α) prepared in step (1) and cholesterol (CHO) were prepared according to the ratio of 69:8 : The molar ratio of 8:15 was placed in a round bottom flask, and Dil was added at the same time. The dosage of Dil is 0.5 mg Dil per 10 mg lipid (lipid includes DPPC, DPPG, DSPE-PEG-COOH-BMS-α and CHO four substances). Then add 5ml of chloroform and 2ml of methanol to fully dissolve. The lipid film was obtained by rotary evaporation for 30 min in an environment with a water bath temperature of 55° C. and a negative pressure of -0.1 MPa.
Embodiment 3
[0079] Embodiment 3: Preparation of phase change nano drug system (PFP@LIPs)
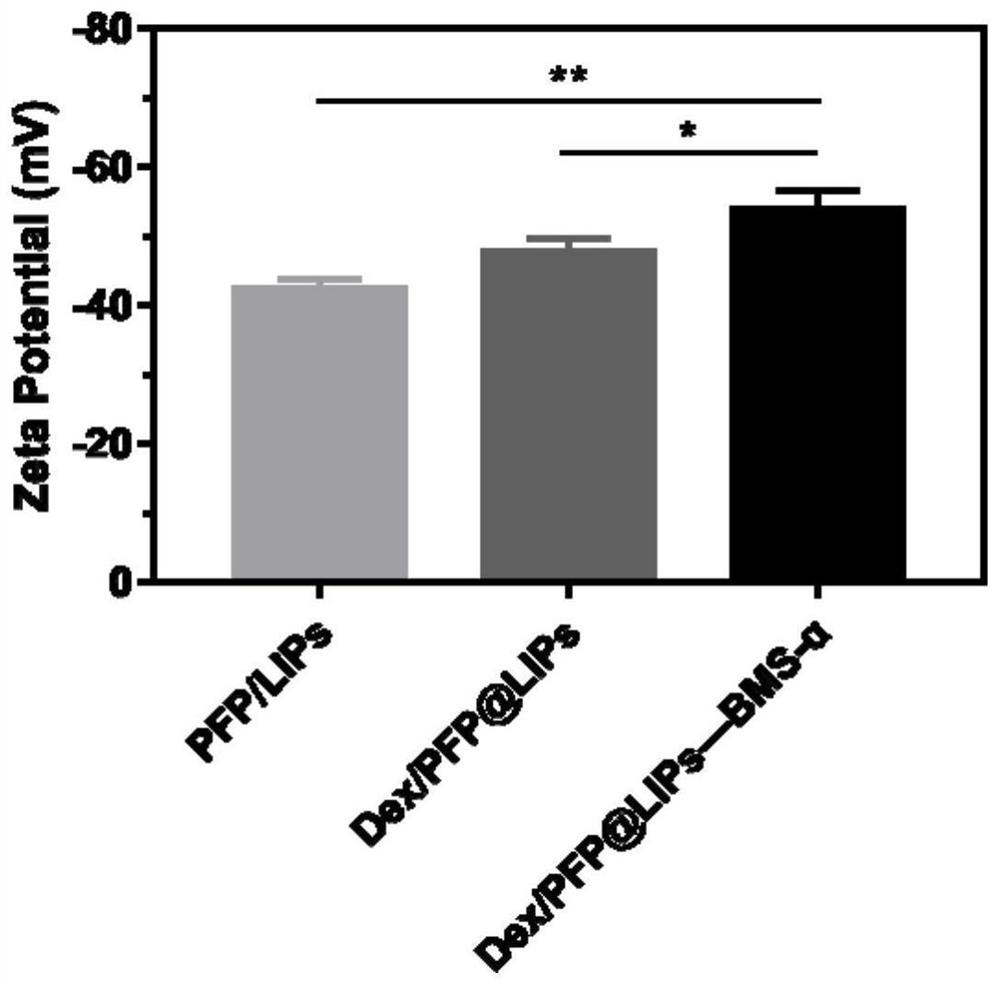
[0080]The present embodiment is basically the same as Example 2, the difference is: do not use DSPE-PEG-COOH-BMS-α (that is, no (1) DSPE-PEG-COOH-BMS-α synthesis), in (2) and (3 ) in the synthesis step, use DSPE-PEG-COOH instead of DSPE-PEG-COOH—BMS-α. Potential analysis was carried out on the PFP@LIPs prepared by this scheme, and the results were as follows image 3 As shown, the average Zeta potential was (-42.3±0.9)mV.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com