Method for detecting cefixime polymer impurity
A technology of cefixime and polymers, which is applied in the field of drug quality testing, and can solve problems such as poor peak shapes of cefixime polymers
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] The preparation of embodiment 1 stock solution
[0064] Preparation of diluent solution: 0.075 mol / L anhydrous disodium hydrogen phosphate: 0.075 mol / L anhydrous sodium dihydrogen phosphate=61:39.
[0065] Sensitivity solution preparation: Accurately measure an appropriate amount of the control solution, dilute it with a diluent to make a solution containing about 0.5 mg of cefixime per 1 ml, and use it as a sensitivity solution.
[0066] Preparation of test solution: get cefixime granules (G1802001 batch), add appropriate amount of diluent, dissolve by ultrasonic, dilute with diluent to make a solution containing cefixime 1mg per 1ml, filter, discard 2.0ml First filtrate, get the continued filtrate as need testing solution;
[0067] Preparation of control solution: Accurately measure an appropriate amount of the test solution, and dilute it with a diluent to prepare a solution containing about 1 μg of cefixime per 1 ml, as a control solution.
[0068] Preparation of ...
Embodiment 2
[0069] Embodiment 2 system suitability test
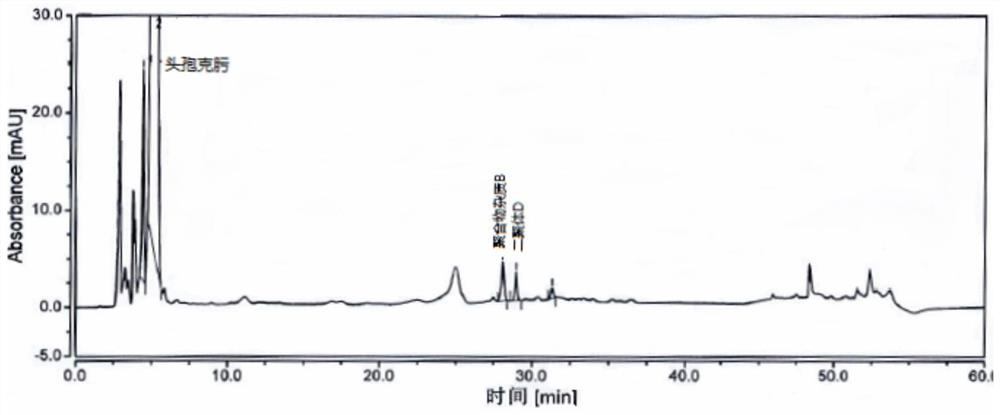
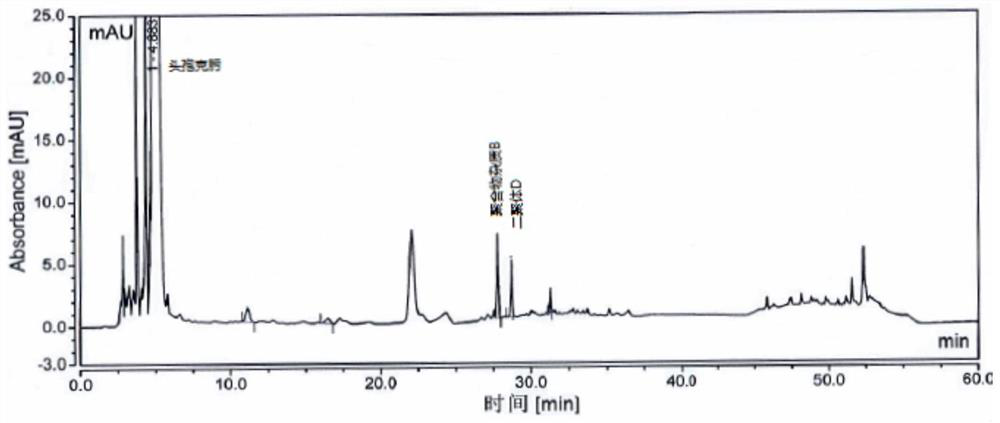
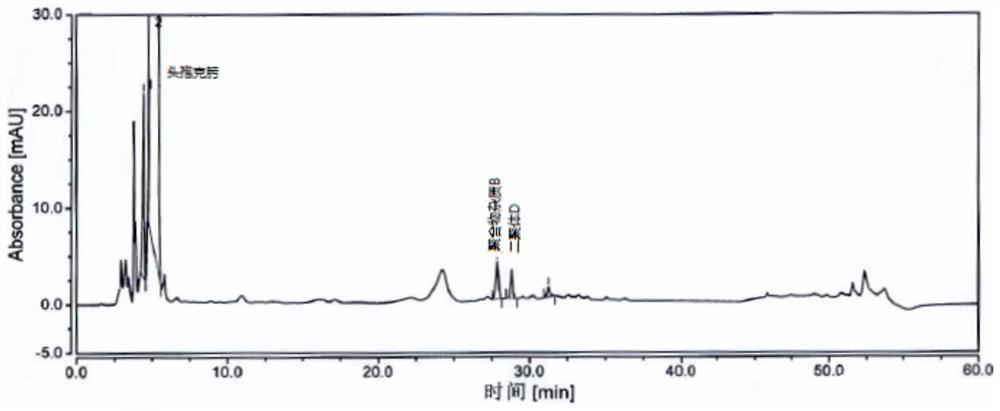
[0070] Take an appropriate amount of cefixime polymer system suitability reference substance, add phosphate buffer (0.075mol / L anhydrous disodium hydrogen phosphate: 0.075mol / L anhydrous sodium dihydrogen phosphate=61:39) to dissolve and dilute to prepare Each 1ml contains about 1mg of cefixime, 2mg of polymer impurity B and 2mg of dimer D, take 20ml and inject it into the liquid chromatograph, record the chromatogram, the separation degree of polymer impurity B and dimer D should meet Require.
Embodiment 3
[0071] The detection of polymer impurity in the cefixime granule of embodiment 3
[0072] Chromatographic column: Hypersil Gold C18, 4.6mm×250mm, 3μm;
[0073] Mobile phase: mobile phase A is 0.1% formic acid solution, and mobile phase B is acetonitrile.
[0074] Flow rate: 1.0ml / min;
[0075] Detection wavelength: 254nm;
[0076] Column temperature: 35°C;
[0077] Injection volume: 20 μL.
[0078] Gradient elution program: as shown in Table 3.
[0079] Table 3 Gradient elution program
[0080]
[0081]
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com