Fullerene nanoparticles for enhancing antitumor immunotherapy
An anti-tumor immunity and nanoparticle technology, applied in the field of fullerene nanoparticles, can solve problems such as cytotoxicity and achieve high tumor inhibition rate, enhanced therapeutic effect, and significant effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0083] The present disclosure also relates to the preparation method of the fullerene nanoparticle, the amino acid-modified fullerene water-soluble modification obtained by modifying the fullerene or metal fullerene with a hydroxyl group and a water-soluble amino acid.
[0084] In a specific embodiment, the fullerene solid powder is reacted with the water-soluble amino acid under alkaline solution, or the organic solution of fullerene is reacted with the water-soluble amino acid alkaline solution under the phase transfer catalyst.
[0085] In a specific embodiment, the preparation method includes the following steps:
[0086] 1) prepare the alkali solution of water-soluble amino acid;
[0087] 2) Add the alkali solution in step 1) into the container filled with fullerene solids and stir for reaction.
[0088] Preferably, the mass fraction of alkali in the alkali solution is 15-60%; more preferably 28-40%;
[0089] Preferably, the molar ratio of the water-soluble amino acid t...
Embodiment 1
[0105] Embodiment 1: Preparation of water-soluble amino acid metallofullerene
[0106] 1) Add 100mg Gd@C 82 Add the solid powder (purchased from Xiamen Funa New Material Technology Co., Ltd.) into a 100ml single-mouth bottle, add 7.2g of β-alanine and 100mL of 14% sodium hydroxide aqueous solution respectively, heat the oil bath to 80°C, and react 4- 7 hours.
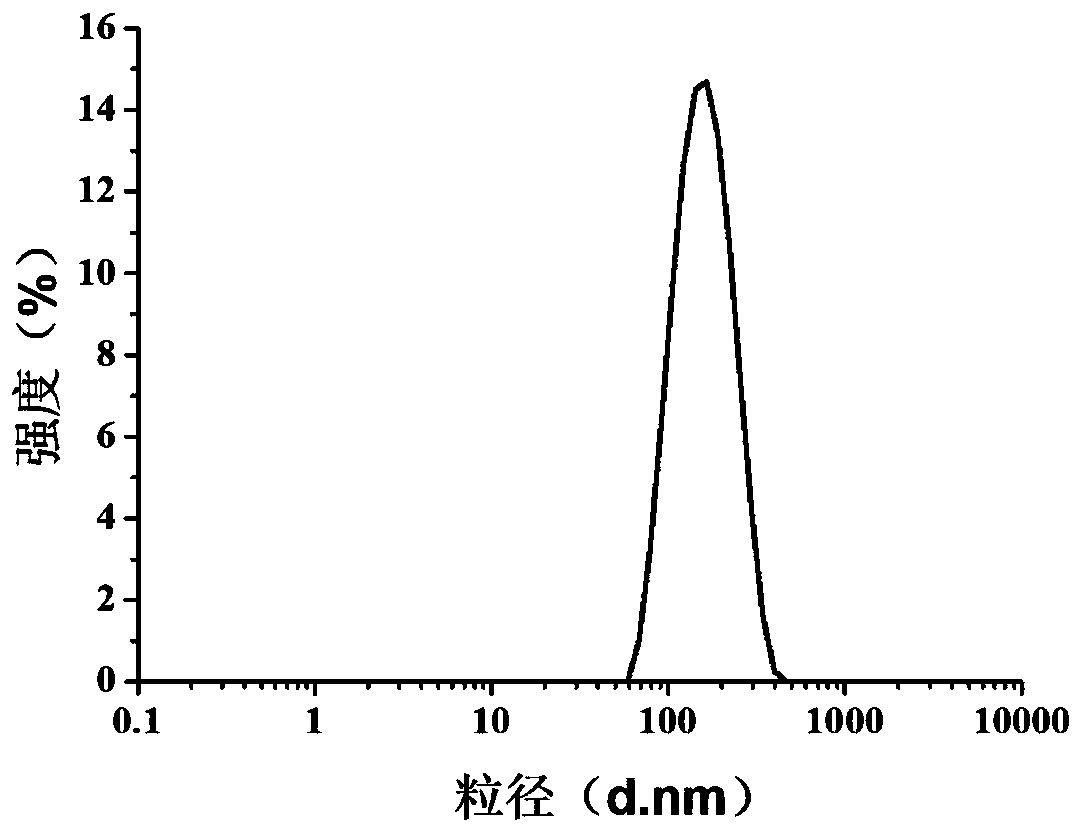
[0107] 2) After the reaction, use a M.W.=3500 dialysis bag to remove small molecules, use a conductivity meter to monitor the conductivity until the dialysis is completed, and concentrate the obtained product to obtain alanine-modified gadolinium metallofullerene hydroxylated modified (GF-Ala ), its average particle diameter in aqueous solution measured by dynamic light scattering (DLS) is 144nm ( figure 1 ), with a uniform particle size distribution.
Embodiment 2
[0108] Example 2: Tumor immunotherapy effect of water-soluble amino acidated metal fullerene
[0109] 1) Induction of water-soluble amino acidated metallofullerenes on the polarization of macrophages
[0110] Cells: RAW264.7 cell line
[0111] Grouping: blank control group, induction group (IL-4), single administration group (GF-Ala) and induction administration group (IL-4+GF-Ala)
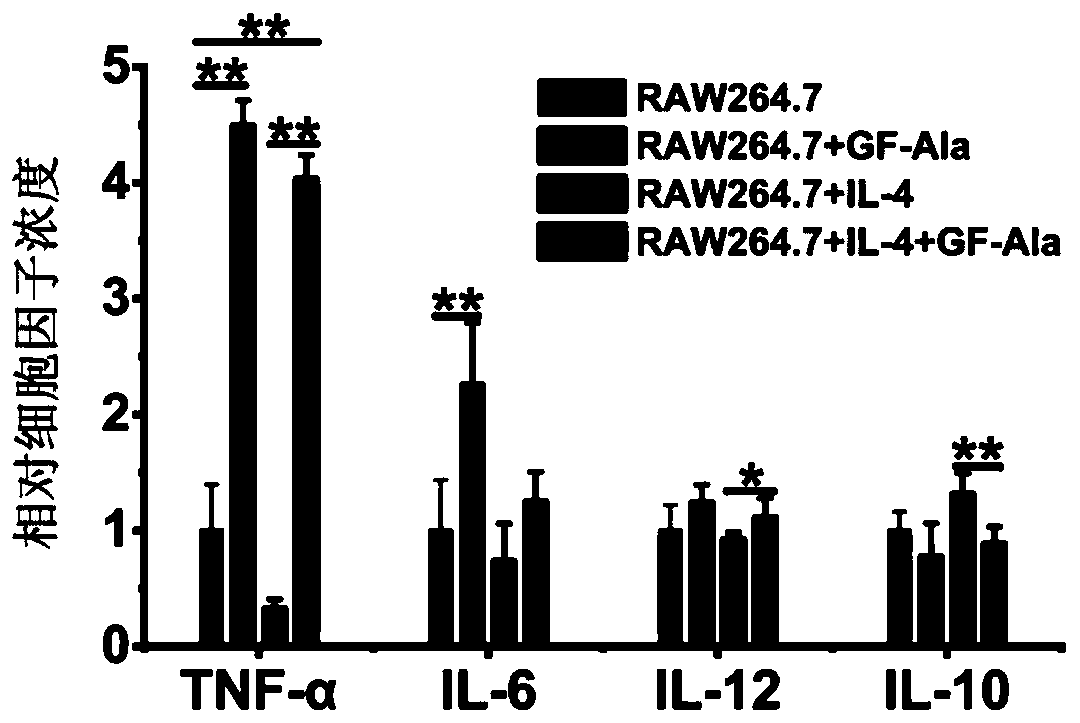
[0112] (1) After the RAW264.7 cell line was in a stable culture state in vitro, IL-4 was induced by group. After 24 hours, the supernatant was aspirated, washed with PBS, and GF-Ala (50 μM) or PBS was added according to the group. The culture was continued for 24 hours. In the supernatant, the levels of cytokines such as TNF-α, IL-6, IL-12 (M1-related cytokine) and IL-10 (M2-related cytokine) were detected by enzyme-linked immunosorbent assay kit (ELISA).
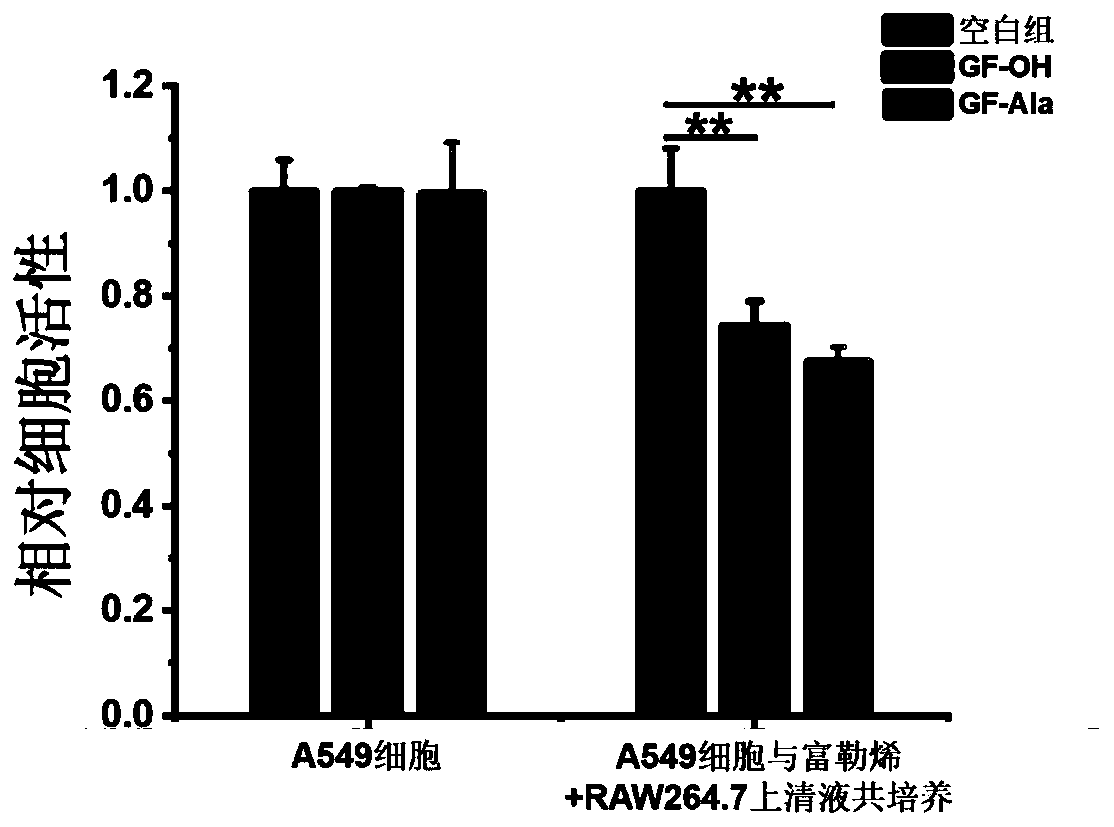
[0113] (2) ELISA test results showed ( figure 2 ), the fullerene treatment group can significantly increase the content of M1-related cyto...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
| Weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com