A kind of electrolyte solution for rechargeable zinc ion battery, its preparation method and rechargeable zinc ion battery
A zinc-ion battery and electrolyte technology, applied in secondary batteries, aqueous electrolytes, circuits, etc., can solve the problems of low electrolyte voltage window, zinc anode dendrite growth, short cycle life and other problems, and achieve good cycle rate performance, The effect of preventing dendrite growth and improving cycle life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Mix 0.5 mL deionized water and 0.5 mL DEC solvent, add 0.73 g Zn(OTf) to the mixed solution 2 Zinc salt, fully oscillated, ultrasonic at room temperature for 10 minutes to completely dissolve the zinc salt, and mix the two solvents completely to obtain 2 mol / kg Zn(OTf) 2 -H 2 O / DEC (1:1) electrolyte.
[0044] Gained electrolyte is carried out following performance test:
[0045] (1) Flame retardant performance
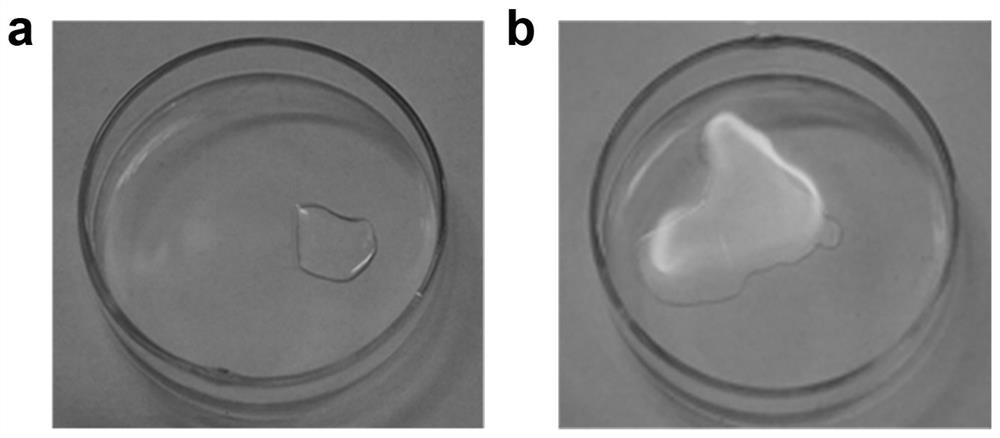
[0046] Ignition test: use a lighter to ignite the electrolyte prepared in this embodiment, and observe its combustion. The result is as figure 1 As shown in a, it shows that the electrolyte prepared in this example cannot be ignited by an open flame at all, and has a good flame retardant effect.
[0047] (2) Conductivity
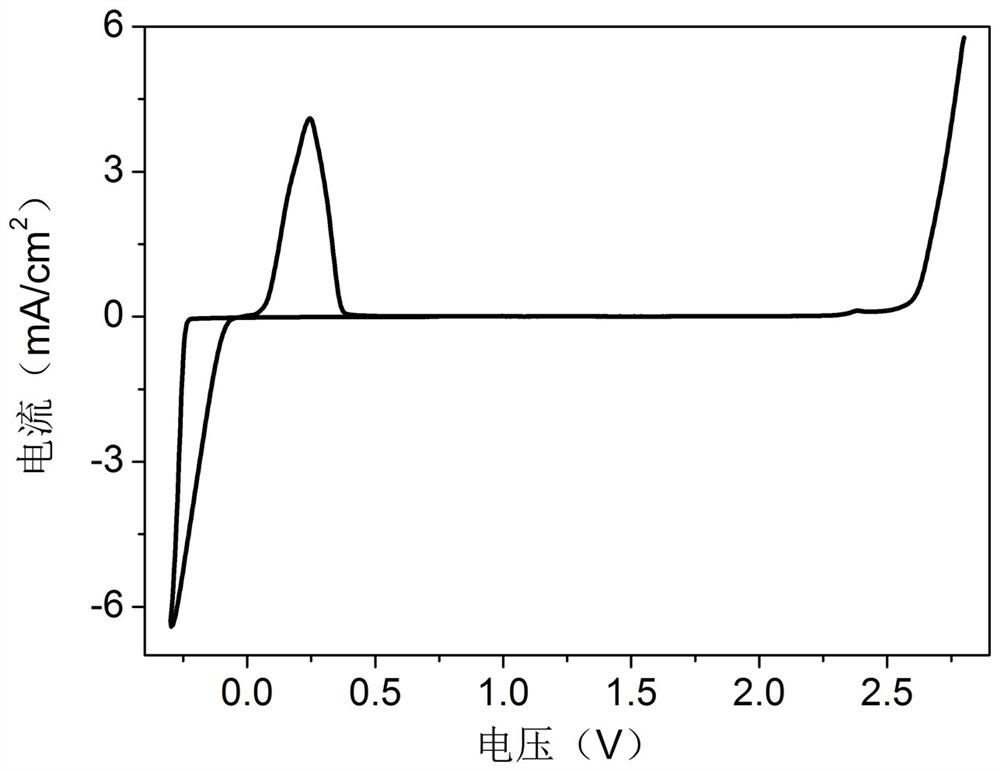
[0048] The conductivity of the electrolyte prepared in this example was tested by the AC impedance method, and the experimental data were recorded on a CHI660E electrochemical workstation. The results show that the conductivity of the e...
Embodiment 2
[0064] Mix 0.7 mL deionized water and 0.3 mL DEC solvent, add 0.73 g Zn(OTf) to the mixed solution 2 Zinc salt, fully oscillate, ultrasonic for 2 minutes at room temperature to completely dissolve the zinc salt, and mix the two solvents completely to obtain 2 mol / kg Zn(OTf) 2 -H 2 O / DEC (7:3) electrolyte.
[0065] The obtained electrolyte was tested for flame retardancy, thermal stability, electrical conductivity and electrochemical performance. The test results show that the electrolyte is non-combustible, the conductivity is as high as 40 mS / cm, and the voltage window is stable from -0.3 V to 2.2 V. The initial coulombic efficiency of the deposition / precipitation of zinc ions in the electrolyte on the zinc anode was 87%, and then stabilized to about 96%, which can be achieved at 1 mA / cm 2 Stable cycle for more than 30 cycles under the current density.
Embodiment 3
[0067] Mix 0.5 mL deionized water and 1 mL DEC solvent, add 1.1 g Zn(OTf) to the mixed solution 2 Zinc salt, fully oscillated, ultrasonic at room temperature for 10 minutes to completely dissolve the zinc salt, and mix the two solvents completely to obtain 2 mol / kg Zn(OTf) 2 -H 2 O / DEC (1:2) electrolyte.
[0068] The obtained electrolyte was tested for flame retardancy, thermal stability, electrical conductivity and electrochemical performance. The test results show that the electrolyte is nonflammable, and the increase in the content of carbonate solvent reduces the conductivity of the electrolyte to 14 mS / cm, and the voltage window is stable from -0.3 V to 2.6 V. The initial coulombic efficiency of the deposition / precipitation of zinc ions in the electrolyte on the zinc anode is 75%, the coulombic efficiency of the first 30 cycles is low between 85 and 90%, and then stabilizes to about 98%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| electrical conductivity | aaaaa | aaaaa |
| electrical conductivity | aaaaa | aaaaa |
| electrical conductivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com