Method for industrially synthesizing monosialotetrahexosyl ganglioside
A technology of ganglioside and monosialic acid, which is applied in the field of industrial synthesis of monosialotetrahexosylganglioside, can solve the problems of low conversion rate, long synthesis cycle, and many steps, and achieve short cycle and simple steps , low cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
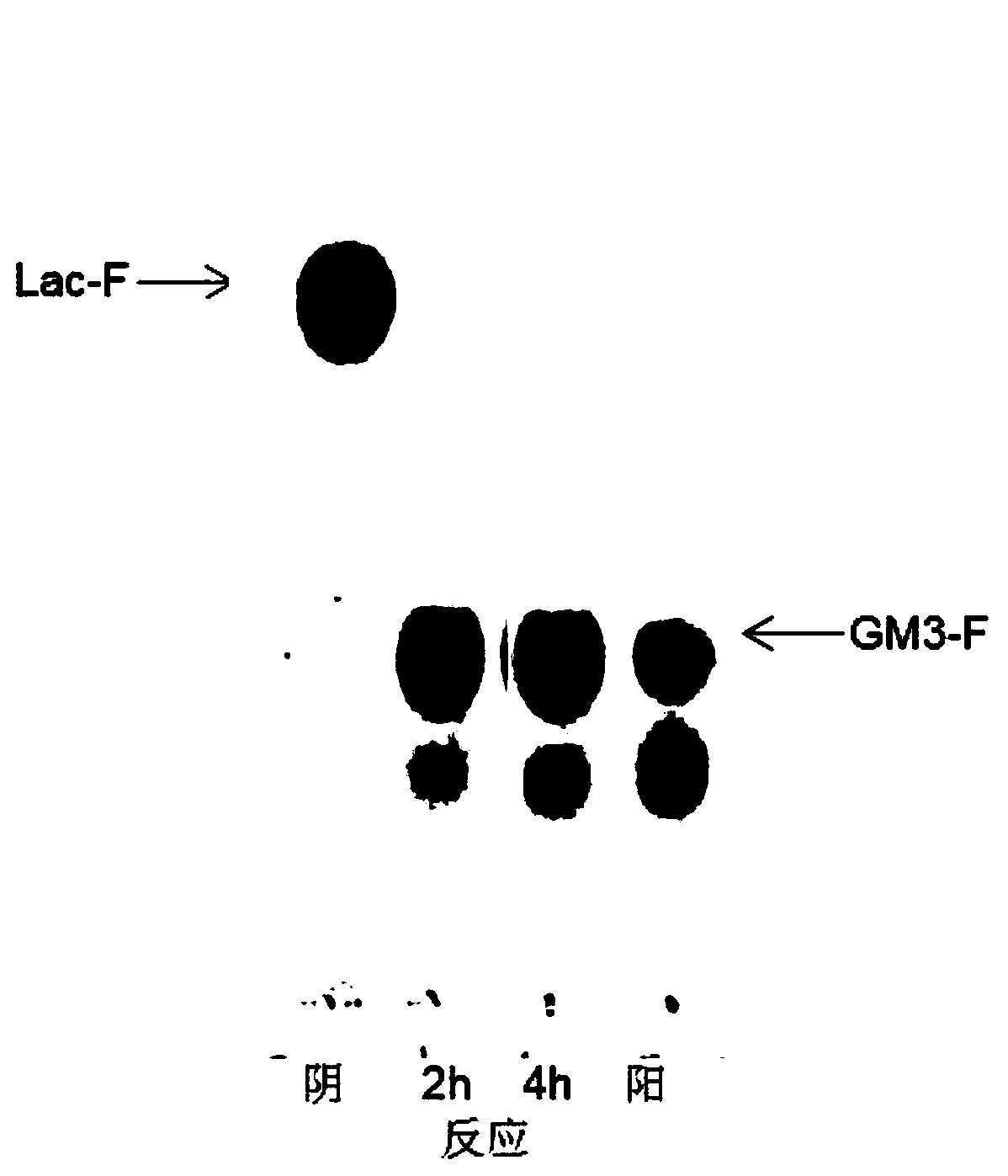
[0089] Step 1: Add 10.23g of fluorinated lactose, 12.98g of pentasodium sialate, 26.85g of disodium cytidine triphosphate and 6.09g of magnesium chloride hexahydrate in sequence in a 2L fermenter, and adjust the pH to 9 with sodium hydroxide. Then add 100U NmCSS, 172UPmST1 and 8.8mg PmPPA, add water to make up to 800mL. React at 30°C for 4 hours, and detect the consumption and production of Lac-F and GM3-F by TLC. For TLC detection, see figure 2 .
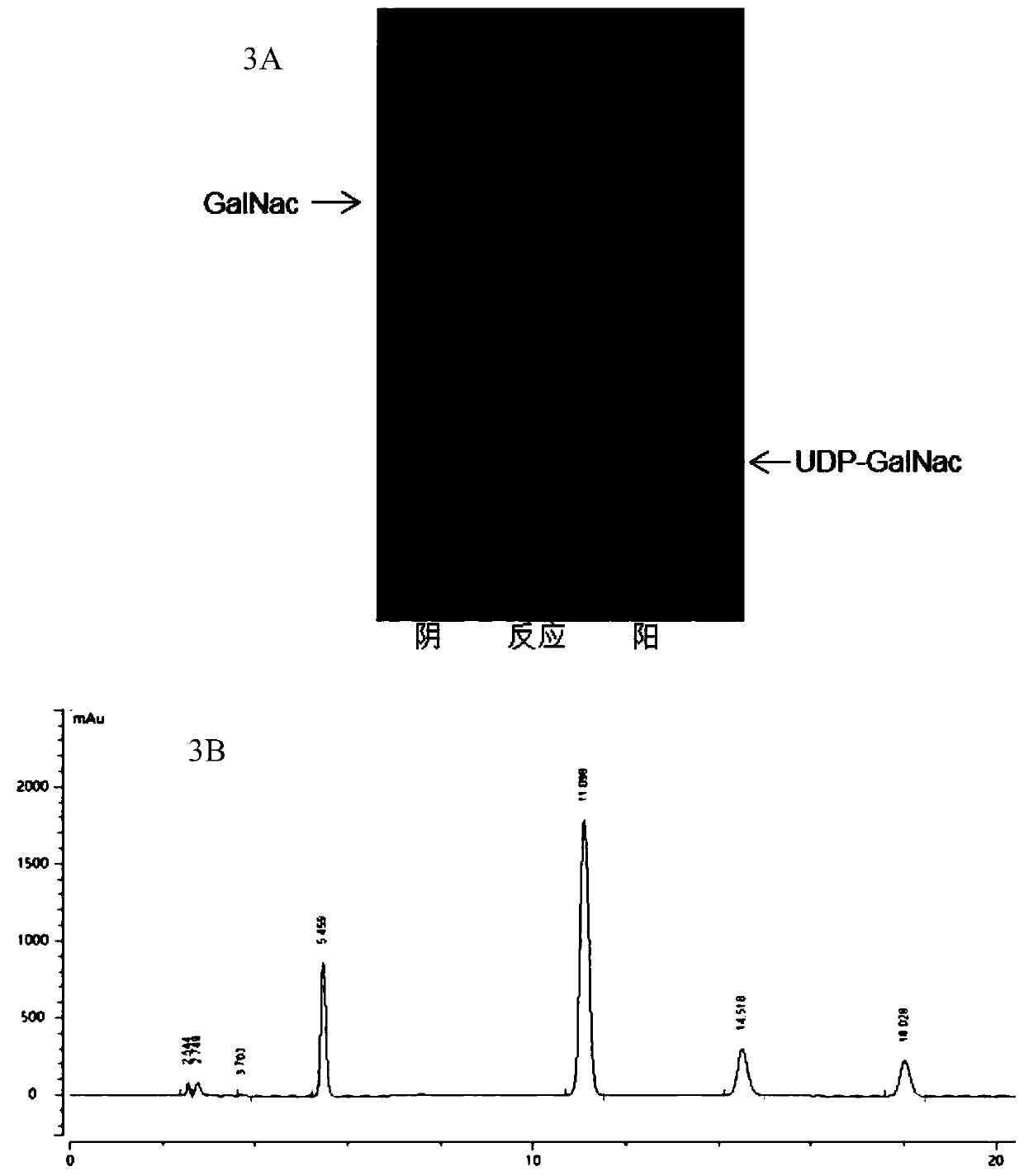
[0090] Step 2: Add 6.63g N-acetylgalactosamine (GalNac), 21.49g adenosine triphosphate disodium, 19.8g uridine triphosphate trisodium and 6.09g magnesium chloride hexahydrate successively in another 2L fermenter, adjust with sodium hydroxide pH to 8. Then add sodium hydroxide to adjust the pH to 8, add 240U NahK, 170U Agx1, 8.8mg PmPPA and add water to make up to 1mL, react at 37°C for 4h, and check the reaction progress by TLC, see image 3 A, HPLC quantification of UDP-GalNac, see image 3 b. Mix the obtained reaction solut...
Embodiment 2
[0093] Embodiment 2: purify GM1-Sph
[0094] The reaction solution in step 4 was adjusted with 1M NaOH, and the pH was adjusted to 7.5; then 800 mL of ethanol was added, settled overnight and then centrifuged. The supernatant was statically adsorbed with 1L ion exchange packing DEAE for 2h. Take out the supernatant, and use a membrane machine to pass through the membrane: the pressure is 8-9kg, and the membrane: 300-500D polyamide composite membrane. Ultrafiltration until the solution is completely dry, add 400mL of absolute ethanol to a 2000mL beaker, and then ultrafiltration until completely dry, repeat twice, add 100mL of water to wash out the product, filter through a 0.22μm membrane, and obtain about 50mL of the solution. Add the aqueous solution obtained in the previous step into a 120g Fresh column with a filler of 50μm tC18, and the elution conditions are: 500mL 100% water—500mL 50% methanol water—1200mL 80% methanol water—500mL 100% methanol. The fraction containing...
Embodiment 3
[0095] Embodiment 3: GM1-Sph high performance liquid phase detection
[0096] Dissolve 1 mg of the white solid obtained in the previous step in 1 mL of aqueous solution, and pass through a 0.22 μm membrane. After treatment, it was detected by high performance liquid chromatography. The elution conditions are shown in Table 1, and the detection conditions are:
[0097] High performance liquid chromatography: Unimicro ChromStation, chromatographic column: Ultimate XB-C18 (Yuexu, 4.6×250mm, 3.5μm), mobile phase A: isopropanol:methanol:acetonitrile=35:40:25; mobile phase B: Water; flow rate: 0.8mL / min; temperature: 40°C, column temperature: 40°C, detector: evaporative light scattering (flow: 2.50L / min).
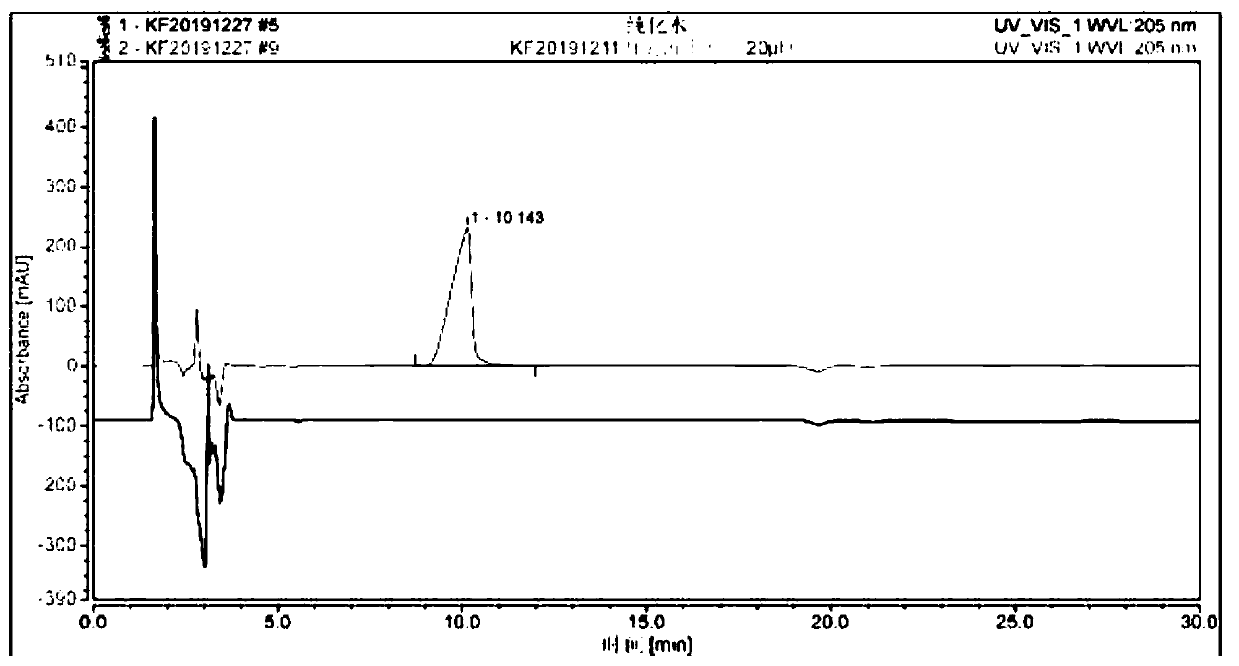
[0098] HPLC results before and after purification see Figure 8 , The retention time of GM1-Sph is 9.5min, the purity is greater than 99%, and the recovery rate is 90%.
[0099] Table 1: Elution Conditions
[0100]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com