Method for recovering acid liquor and separating vanadium in process of reducing acid leaching vanadium-containing waste catalyst
A technology for waste catalysts and catalysts, applied in the direction of improving process efficiency, can solve the problems of low leaching rate, only reaching 85%, low product purity, etc., and achieve the effects of mild leaching conditions, low leaching temperature, and high vanadium leaching rate.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
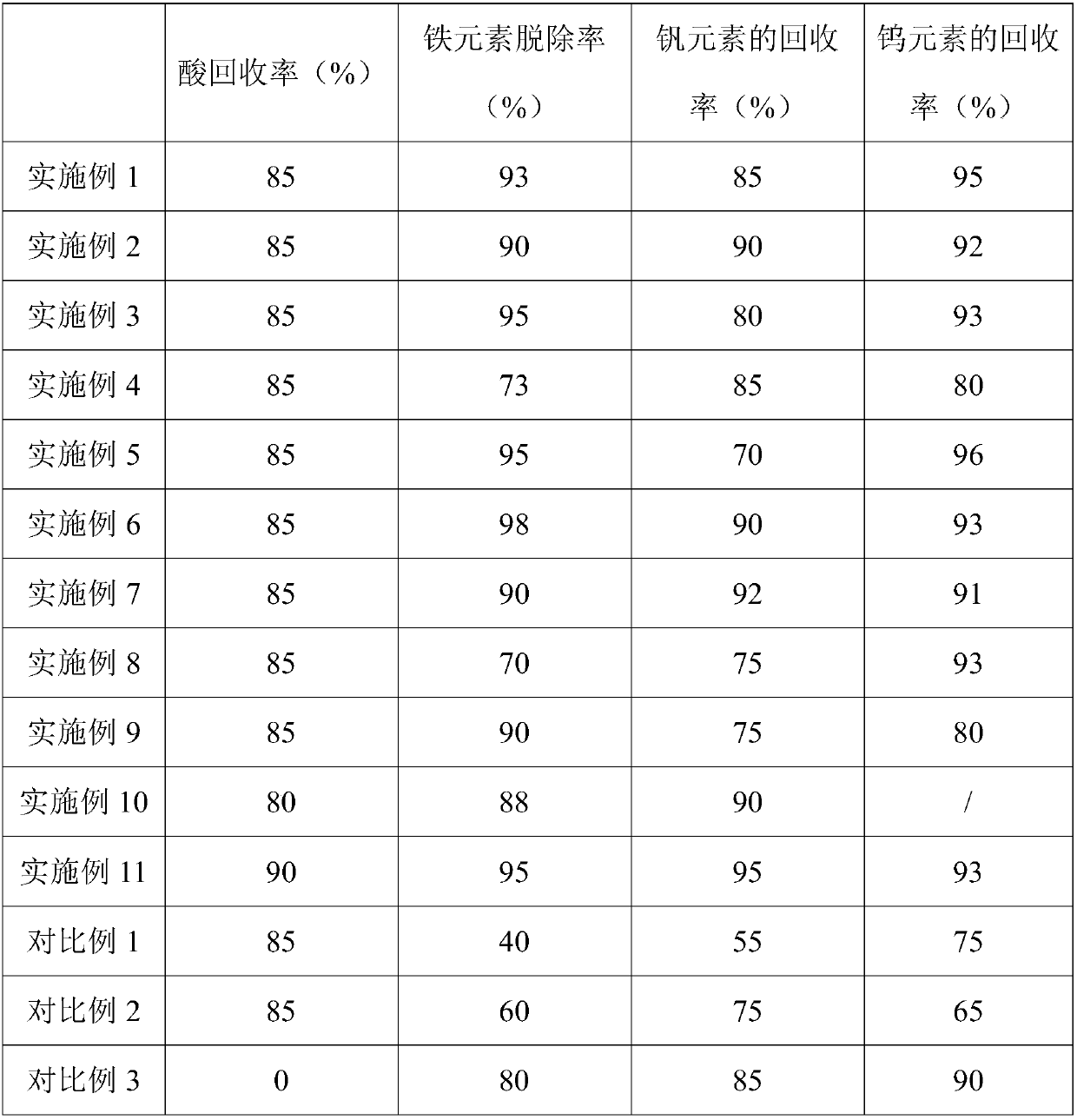
Examples
Embodiment 1
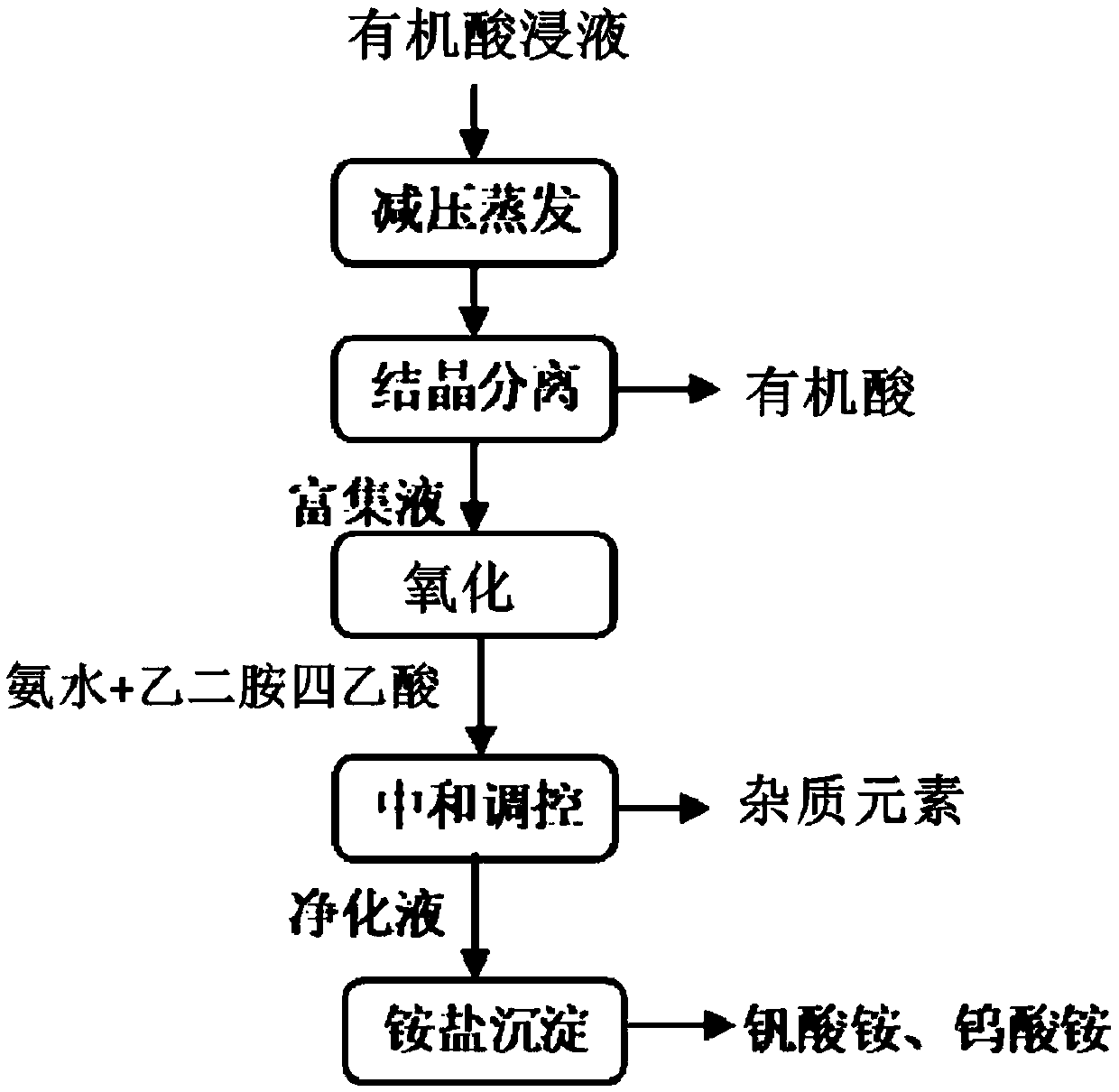
[0064] A method for recovering acid solution and separating vanadium in the process of reductive acid leaching waste catalyst containing vanadium comprises the following steps:
[0065] (1) Add the vanadium-tungsten-titanium-aluminum system catalyst to oxalic acid for leaching at 70°C for 6 hours to obtain an organic acid immersion solution with a vanadium element content of 4.5g / L, and evaporate water under reduced pressure at 80°C in the organic acid immersion solution , the evaporation volume is 80% of the volume of the organic acid immersion solution, then crystallize oxalic acid at 0°C for 8 hours, and filter the oxalic acid crystals to obtain the enrichment solution;
[0066] (2) According to the volume ratio of the enriched solution and hydrogen peroxide as 10:1, mix the enriched solution with hydrogen peroxide, oxidize at 70°C, add ammonia water to the oxidized enriched solution to adjust the first pH value to 3, Add 1g of ethylenediaminetetraacetic acid, carry out the...
Embodiment 2
[0068] The difference from Example 1 is that step (2) adjusts the first pH value to 2.
Embodiment 3
[0070] The difference from Example 1 is that step (2) adjusts the first pH value to 4.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com