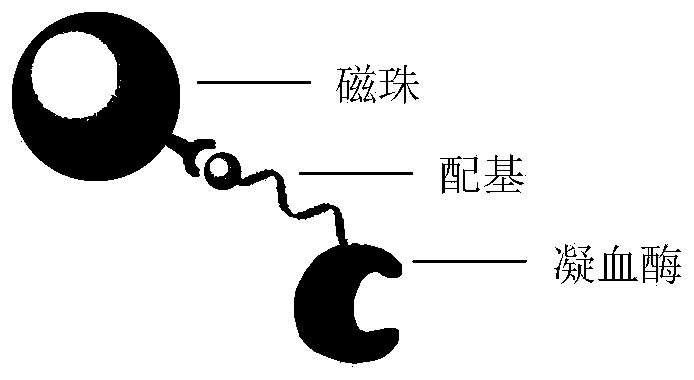
Probe for capturing hirudin polypeptide and application of probe
A technology of hirudin and probe, which is applied in the preparation method of peptides, from leech inhibitors, peptides, etc., can solve the problems of low quantitative sensitivity, poor quantitative repeatability, and difficult detection by time method, and achieve good application prospects and high efficiency. high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Preparation of Example 1 probe (3 μm polystyrene carboxyl-ligand-bovine thrombin)
[0041] Step 1: Conjugate streptavidin. 50mg polystyrene carboxyl magnetic beads (3μm, figure 1 ), 5mg EDC and 1mg streptavidin were dispersed in 1mL MES buffer solution, rotated and mixed at 30°C, reacted for 6 hours, separated the reaction product with a magnet, redispersed into 0.1% BSA solution, and sealed for 6 hours , and washed 5 times with PBS buffer to prepare 3 μm streptavidin-modified polystyrene magnetic beads.
[0042] The polystyrene magnetic beads refer to the cores of porous polystyrene microspheres, the magnetic particles are embedded in the pores, the outer layer is coated with polyacrylic acid, and carboxyl groups are distributed on the surface.
[0043] Step 2: Modify the thrombin ligand. The magnetic beads prepared in step 1 were mixed with the ligand at a ratio of 50 mg: 1 μmol, dispersed in PBS buffer, shaken at room temperature for 1 hour, and the product was w...
Embodiment 2
[0046] Preparation of Example 2 Probe (10 μm agarose carboxyl-ligand-bovine thrombin)
[0047] Step 1: Conjugate streptavidin. 100mg agarose carboxyl magnetic beads (10μm, figure 2 ), 5mg EDC and 2mg streptavidin were dispersed into 1mL MES buffer solution, rotated and mixed at 30°C, reacted for 6 hours, separated the reaction product with a magnet, redispersed into 0.1% BSA solution, and blocked for 6 hours Finally, wash with PBS buffer 5 times to prepare 10 μm streptavidin-modified agarose magnetic beads.
[0048] The agarose carboxyl magnetic beads refer to magnetic beads with carboxyl groups sealed by agarose.
[0049] Step 2: Modify the thrombin ligand. Mix the magnetic beads prepared in step 1 with the ligand at a ratio of 100 mg: 2 μmol, disperse them in PBS buffer, shake at room temperature for 1 hour, wash the product with deionized water 5 times, and obtain 10 μm ligand-modified agarose magnetic beads .
[0050] The thrombin ligand is as follows: 5'biotin-TTTTTTT...
Embodiment 3
[0052] Preparation of Example 3 Probe (25 μm agarose NHS-ligand-bovine thrombin)
[0053] Step 1: Conjugate streptavidin. 100mg agarose NHS magnetic beads (25μm, image 3 ) and 2 mg of streptavidin were dispersed in 1 mL of MES buffer solution, rotated and mixed at 30°C, and reacted for 6 hours. The reaction product was separated with a magnet, re-dispersed into 0.1% BSA solution, and blocked for 6 hours. Wash with PBS buffer 5 times to prepare 25 μm streptavidin-modified agarose magnetic beads.
[0054] The agarose NHS magnetic beads refer to magnetic beads sealed by agarose with NHS groups (belonging to activated carboxyl groups).
[0055] Step 2: Modify the thrombin ligand. Mix the magnetic beads prepared in step 1 with the ligand at a ratio of 100 mg: 2 μmol, disperse in PBS buffer, shake at room temperature for 1 hour, and wash the product with deionized water 5 times to obtain 25 μm ligand-modified agarose magnetic beads .
[0056] The thrombin ligand is as follows:...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com