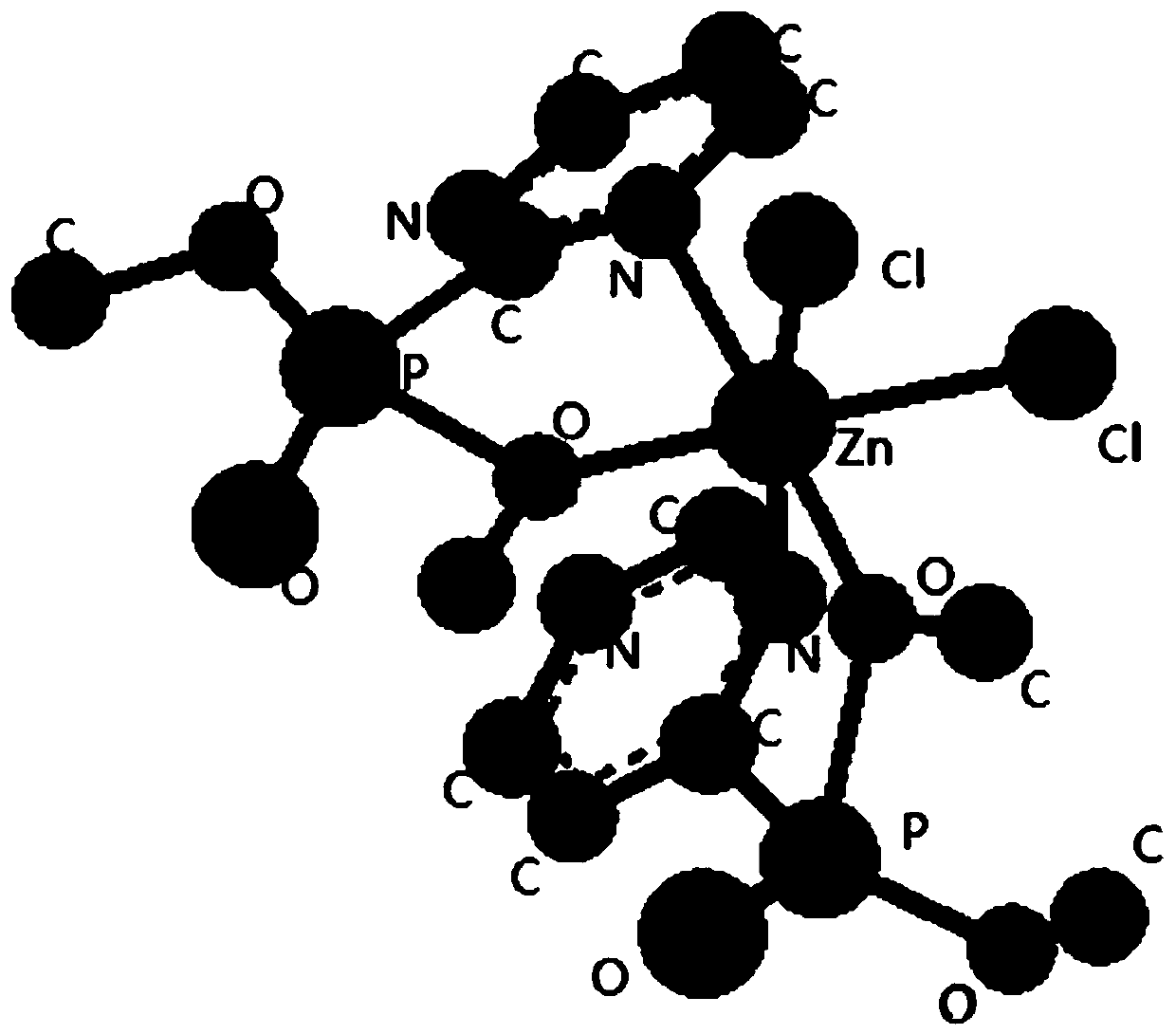
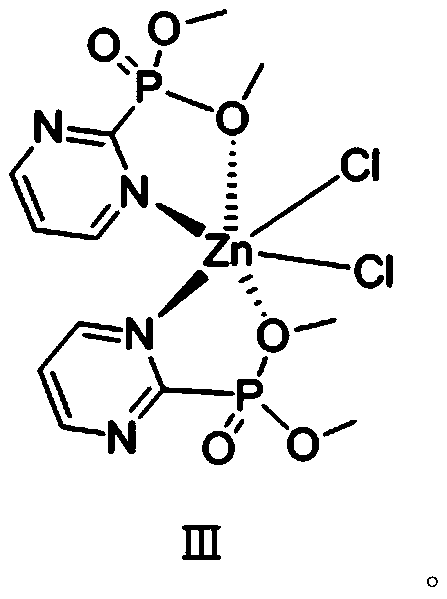
Phosphate-pyrimidine zinc dichloride complex with catalytic performance as well as preparation method and application of phosphate-pyrimidine zinc dichloride complex
A technology of zinc pyrimidine dichloride, catalytic performance, applied in the direction of organic compound/hydride/coordination complex catalyst, organic chemical method, zinc organic compound, etc., can solve the trouble of removing anti-gelling agent and low grafting rate And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0034] The second aspect of the embodiments of the present invention provides a method for preparing a phosphoric acid-pyrimidine zinc dichloride complex with catalytic properties, comprising the following steps:
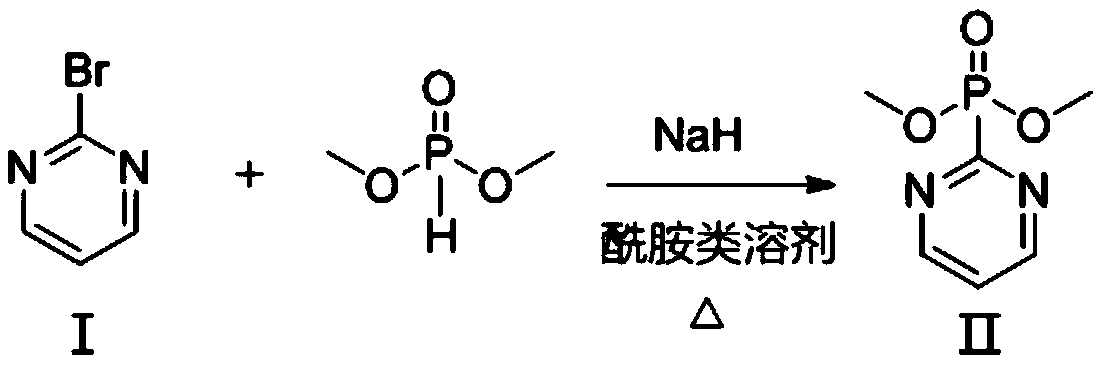
[0035] (1) Synthesis of organic ligand pyrimidine-2 phosphate dimethyl:
[0036]
[0037] Dissolving 2-bromopyrimidine as shown in the structure of formula I in an amide solvent to form a 2-bromopyrimidine solution;
[0038] Suspend sodium hydride in an amide solvent to form a sodium hydride suspension, add dimethyl phosphite to the sodium hydride suspension, stir for reaction, then add the 2-bromopyrimidine solution, heat up the reaction, and wait for the reaction After the end, concentrate under reduced pressure to remove the amide solvent to form a concentrate, add ice water to the concentrate, extract the water phase, combine the organic phase, wash, dry, and concentrate the organic phase to obtain a crude product, purify Process to obtain pyrimidine-2-phosp...
Embodiment 1
[0052] A method for preparing a phosphoric acid-pyrimidine zinc dichloride complex with catalytic performance, comprising the steps of:
[0053] (1) Synthesis of organic ligand pyrimidine-2 phosphate dimethyl:
[0054]
[0055] In a container equipped with magnetic stirring, 4.0g (100mmol) sodium hydride (commercial reagent, sodium hydride dispersed in mineral oil, sodium hydride content in 60wt%) was suspended in 200mL of anhydrous dimethylacetamide ( DMAc) solvent to form a sodium hydride suspension, then slowly add a DMAc solution (10 mL) of 11 g (100 mmol) dimethyl phosphite (CAS No. 868-85-9) into the sodium hydride suspension under stirring, and add After completion, the reaction was stirred at room temperature for 0.5h; then 15.8g (100mmol) of 2-bromopyrimidine in DMAc (50mL) as shown in the structure of formula I was added to the above reaction system, and then the temperature was raised to 160°C for 24h; the reaction ended Finally, the oil pump concentrated under ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com