Synthesis method of 4,7-dichloroquinoline
A technology of dichloroquinoline and synthetic methods, applied in 4 fields, can solve the problems of expensive raw materials, unfavorable industrial production, high cost of raw materials, etc., and achieve the effects of low cost, good product quality and good purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
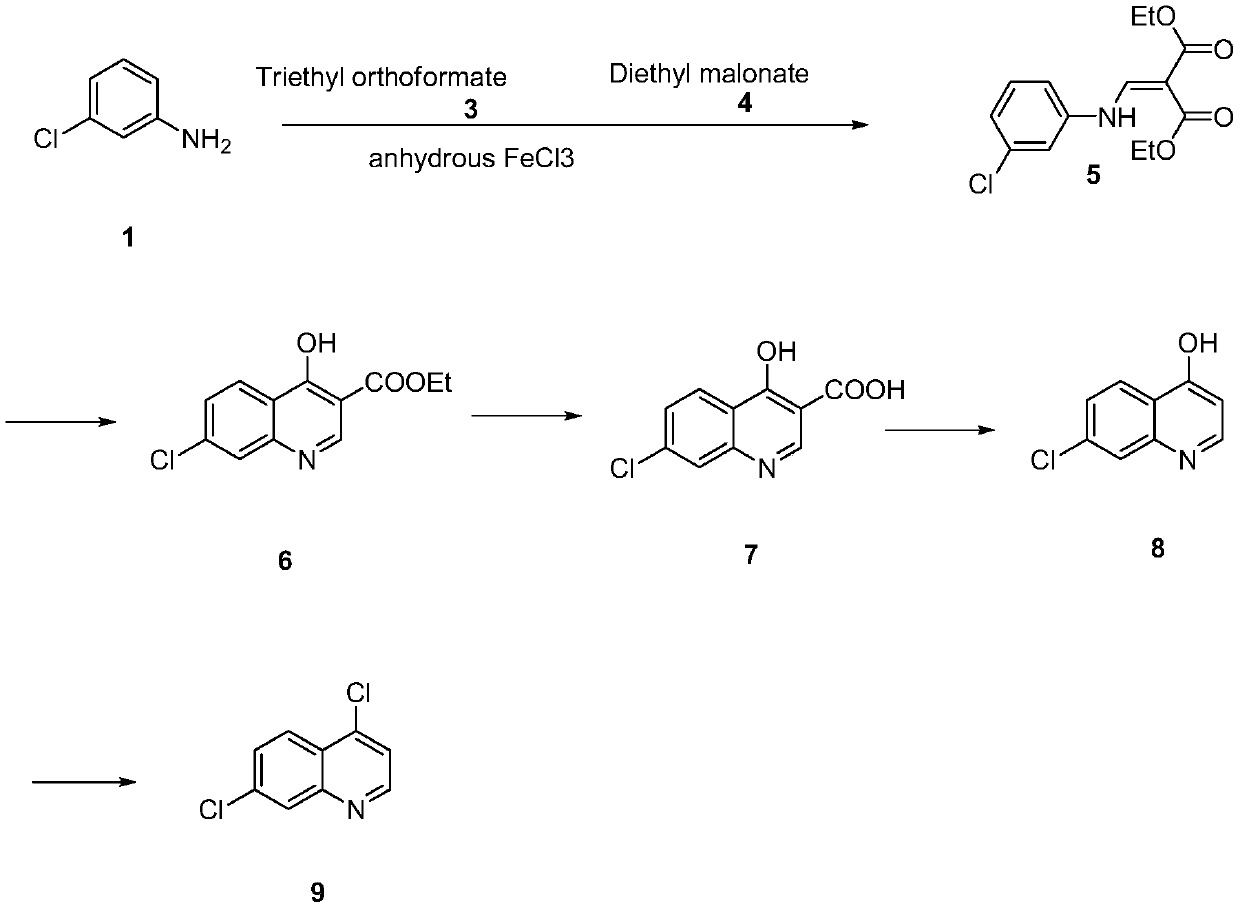
[0021] Synthesis and condensation of diethyl 2-[[(3-chlorophenyl)amino]methylene]malonate (5)
[0022] Add m-chloroaniline (101.6g, 0.8mol), triethyl orthoformate (118.4g, 0.8mol), diethyl malonate (128g, 0.8mol) in a reaction vessel equipped with a stirring and distillation device, without Mix ferric chloride (0.10g) with water evenly. After the addition, the temperature was raised to 110° C. and the reaction was kept for 2 hours. During the reaction, ethanol would evaporate, and after 2 hours, triethyl orthoformate (88.8 g, 0.6 mol) was added dropwise to the reaction system. After adding the material, raise the temperature to 130-140°C, keep the temperature for 5 hours, and then use vacuum distillation to remove the residual ethanol after the reaction. The crude oily product remains, and the crude product directly enters the next step of cyclization.
[0023] Synthesis of Ethyl 7-Chloro-4-Hydroxy-3-quinolinecarboxylate (6) and 4-Hydroxy-7-Chloroquinolinic Acid (7) (one-pot ...
Embodiment 2
[0030] Synthesis and condensation of diethyl 2-[[(3-chlorophenyl)amino]methylene]malonate (5)
[0031] Add m-chloroaniline (101.6g, 0.8mol), trimethyl orthoformate 0.8mol, diethyl malonate (128g, 0.8mol) and anhydrous iron trichloride in a reaction vessel equipped with a stirring and distillation device (0.10g) and mix well. After the feeding is completed, raise the temperature to 120°C and keep it warm for 3 hours. Methanol will evaporate during the reaction. After 3 hours, add 0.8 mol of trimethyl orthoformate dropwise to the reaction system. After adding the material, raise the temperature to 130-140°C, keep it warm for 4 hours, and then use vacuum distillation to remove the residual methanol after the reaction. The crude oily product remains, and the crude product directly enters the next step of cyclization.
[0032] Synthesis of Ethyl 7-Chloro-4-Hydroxy-3-quinolinecarboxylate (6) and 4-Hydroxy-7-Chloroquinolinic Acid (7) (one-pot method of ring closure and hydrolysis) ...
Embodiment 3
[0039]Synthesis and condensation of diethyl 2-[[(3-chlorophenyl)amino]methylene]malonate (5)
[0040] Add m-chloroaniline (101.6g, 0.8mol), triethyl orthoformate (118.4g, 0.8mol), diethyl malonate (128g, 0.8mol) in a reaction vessel equipped with a stirring and distillation device, without Mix ferric chloride (0.15g) with water evenly. After the addition, the temperature was raised to 100° C. and the reaction was kept for 1 hour. During the reaction, ethanol would evaporate, and after 1 hour, triethyl orthoformate (71 g, 0.48 mol) was added dropwise to the reaction system. After adding the material, raise the temperature to 130-140°C, keep the temperature for 5 hours, and then use vacuum distillation to remove the residual ethanol after the reaction. The crude oily product remains, and the crude product directly enters the next step of cyclization.
[0041] Synthesis of Ethyl 7-Chloro-4-Hydroxy-3-quinolinecarboxylate (6) and 4-Hydroxy-7-Chloroquinolinic Acid (7) (one-pot meth...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com