Sustained-release preparation containing piracetam
A technology of sustained-release preparations and piracetam, which is applied to medical preparations containing active ingredients, medical preparations without active ingredients, and pill delivery, which can solve the problems of carrying, inconvenient use, high frequency of administration, and repetitive It is suitable for large-scale promotion and application, with good compliance and less adverse reactions.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
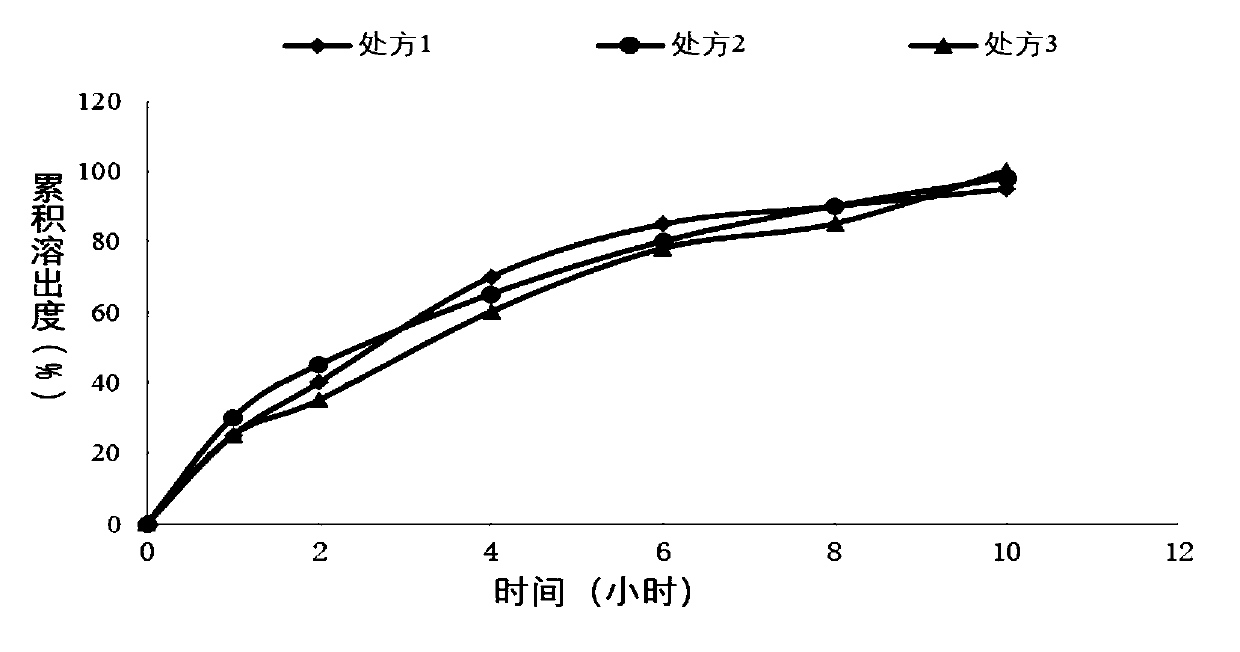
[0049] Sustained-release tablet preparation of different specifications of embodiment 1
[0050]
[0051] Weigh piracetam, hypromellose K100LV, and lactose according to the above formula, place them in a wet granulator, turn on the stirring paddle and the shear paddle, both at low speed, mix for 10 minutes, add purified water to wet granulate, and then Place in a fluidized bed to dry and control the moisture to be no more than 3.0%, granulate with 20 meshes, add magnesium stearate to the dry granules, and compress with a rotary tablet machine. The tablet is film-coated in a high-efficiency coating machine, and the coating solution is ethyl cellulose aqueous dispersion (Surelease) coating, and the coating weight gain is 3-6%. One-sided punching, the finished product after packaging.
Embodiment 2
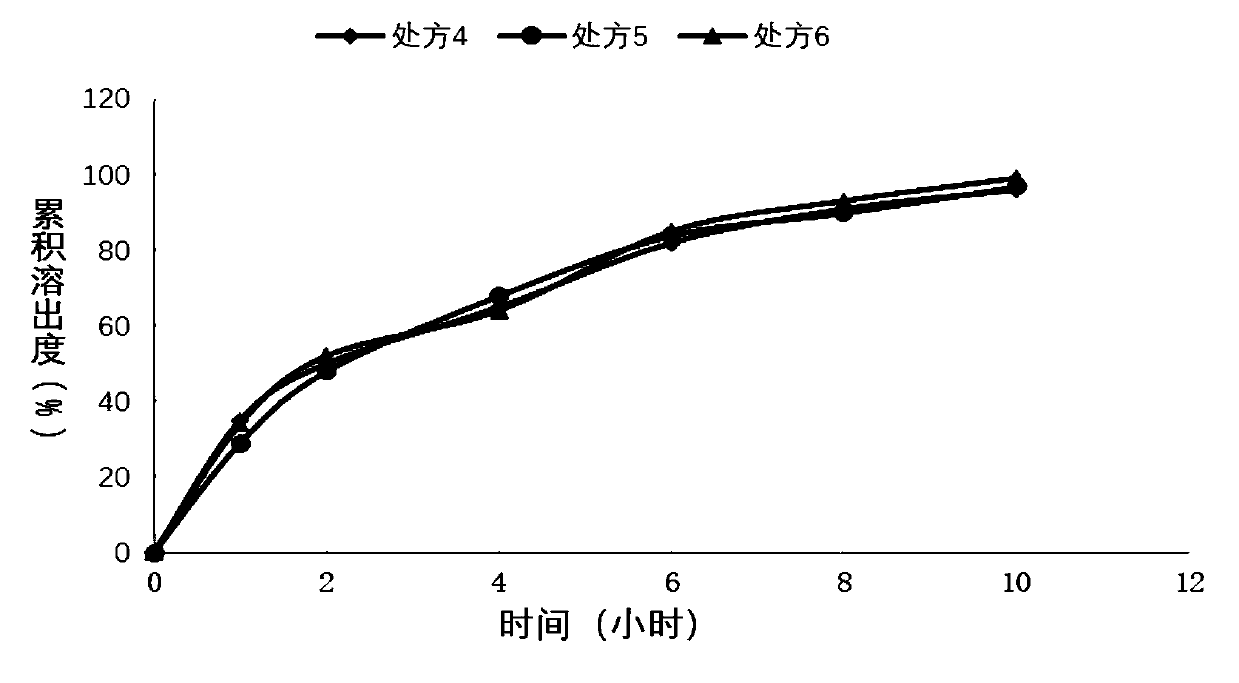
[0052] Sustained-release tablet preparation of embodiment 2 different polymer ratios
[0053]
[0054]Weigh piracetam, hypromellose K100LV, hypromellose E4M, and lactose according to the above formula, place them in a wet granulator, turn on the stirring paddle and shearing paddle, both at low speed, mix for 10 minutes, add Wet granulation with purified water, and then placed in a fluidized bed to dry to control the moisture content not to exceed 3.0%, granulated to 20 mesh, magnesium stearate was added to the dry granules, and tabletted with a rotary tablet machine. Film-coat the tablets in a high-efficiency coating machine. The coating solution is ethylcellulose aqueous dispersion (Surelease) mixed with Opadry at a ratio of 70 / 30 and then coated. The weight gain of the coating is about the weight of the tablet. 5%. The film is perforated on both sides, and the finished product is obtained after packaging.
[0055] The punching in embodiment 1 and embodiment 2 is followi...
Embodiment 3
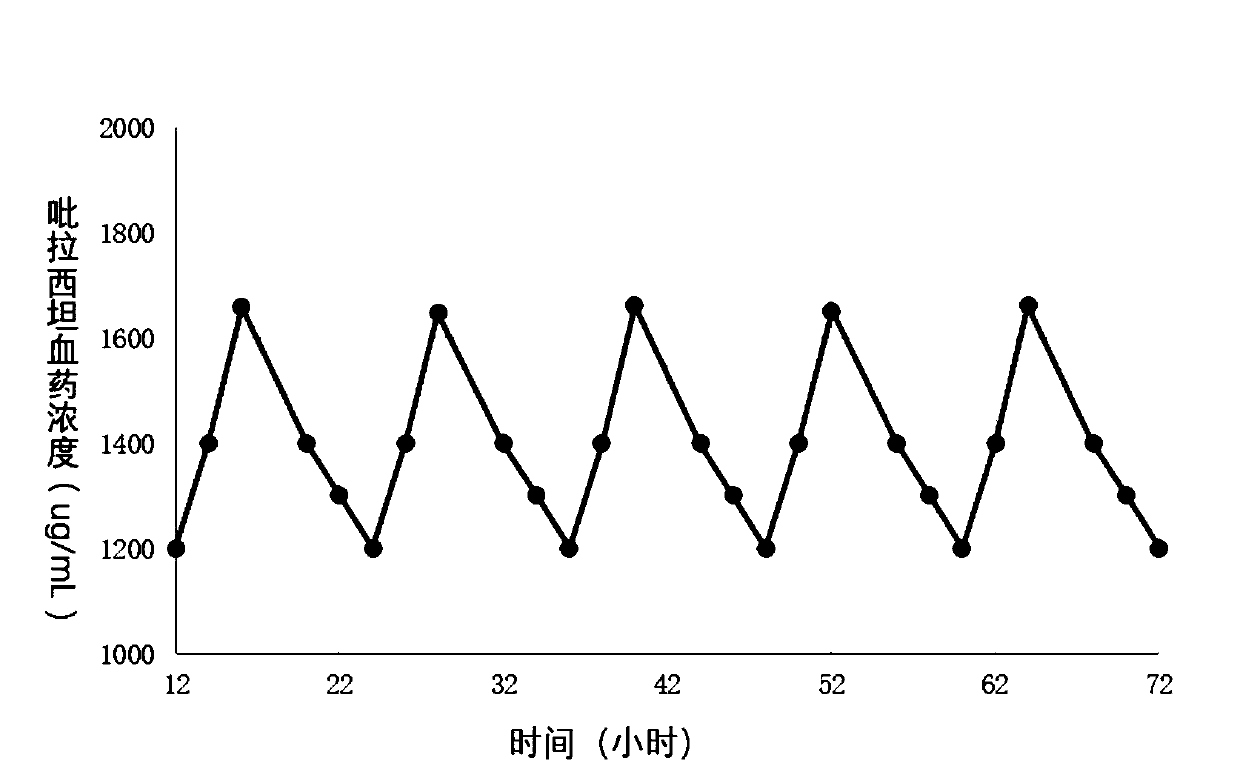
[0057] Example 3: Pharmacokinetics study in Beagle dogs
[0058] 6 healthy Beagle dogs, 3 males and 3 females, with a body weight of 8 to 10 kg, were randomly divided into two groups, each group of 3, and one group carried out release preparation (self-made tablets in embodiment 1, specification 1.2g / sheet , 1 tablet in total) single-dose administration test, the drug dosage is 1.2g / ; , specification: 0.4g / tablet, 3 tablets in total) multiple administration test, administration once every 6 hours, a total of 3 times, the dosage is 0.4g / time / piece, a total of 1.2g / piece. Each Beagle dog took 2ml of blood from the forelimb vein on one side of the dog before taking the medicine (0h), 0.25, 0.5, 1, 2, 3, 4, 5, 6, 8, 12, 16, 24, and 48 hours after taking the medicine. Transfer to a heparinized test tube, centrifuge (5000rpm, 10min), separate the plasma, and freeze it at -20°C until use;
[0059] The concentration of piracetam in plasma was determined by liquid chromatography-tand...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com