Hyaluronic acid cross-linked chondroitin sulfate compound and application thereof
A technology of chondroitin sulfate and sodium hyaluronate, which is applied in the directions of non-active ingredients medical preparations, medical preparations containing active ingredients, and drug combinations, can solve problems such as being easily enzymatically hydrolyzed, and achieve a suitable degree of cross-linking. , the effect of good biological activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
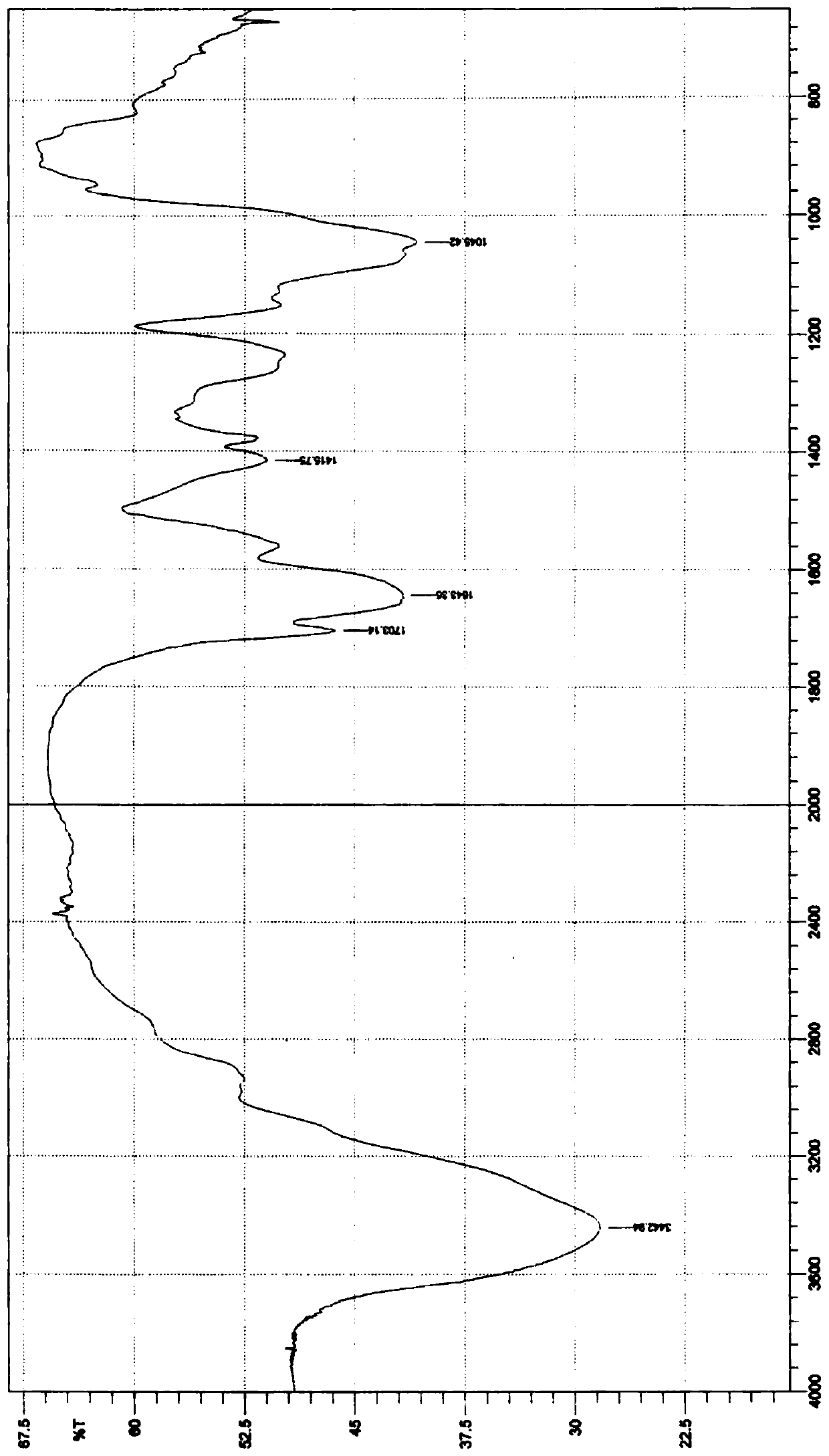
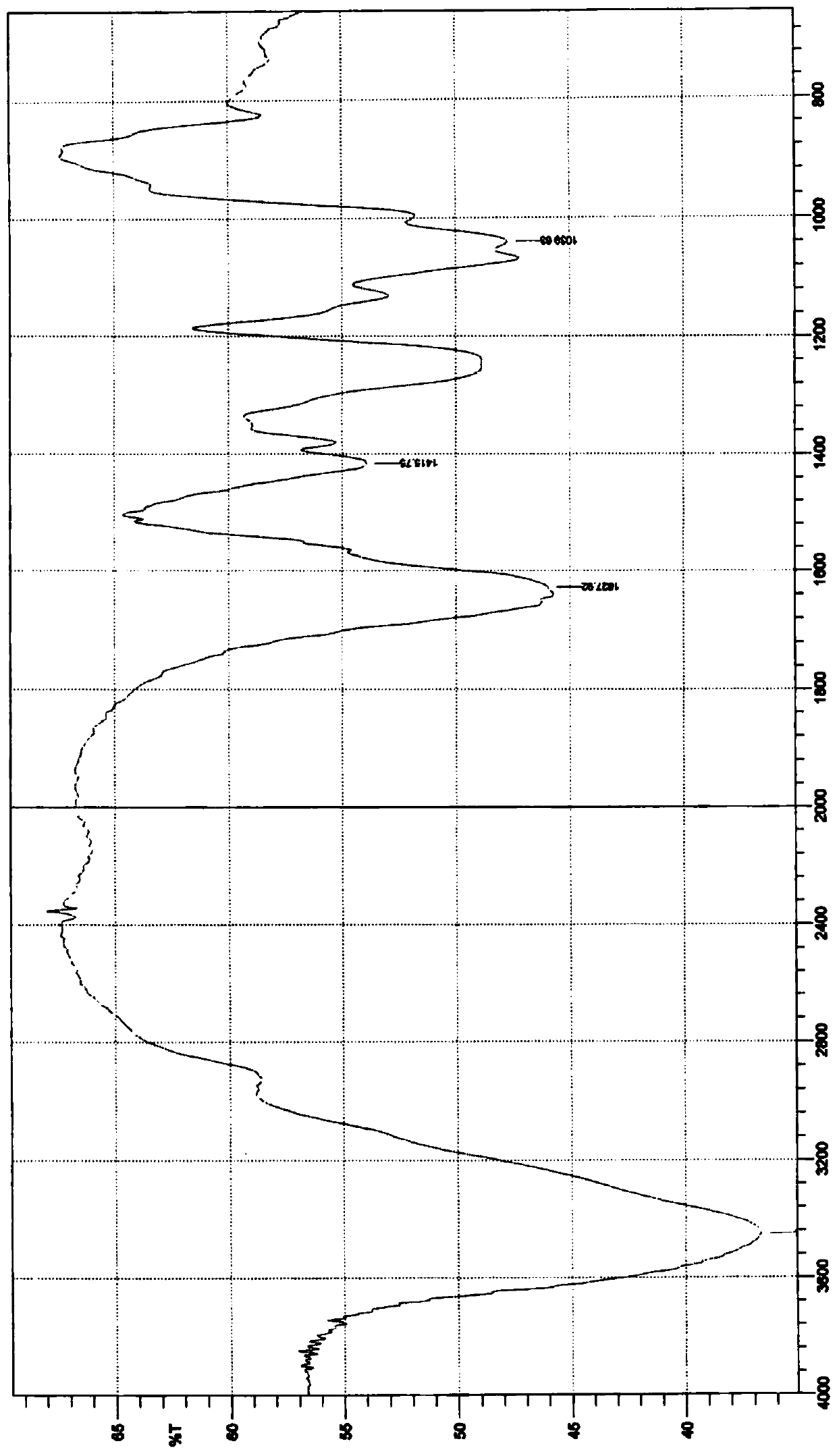
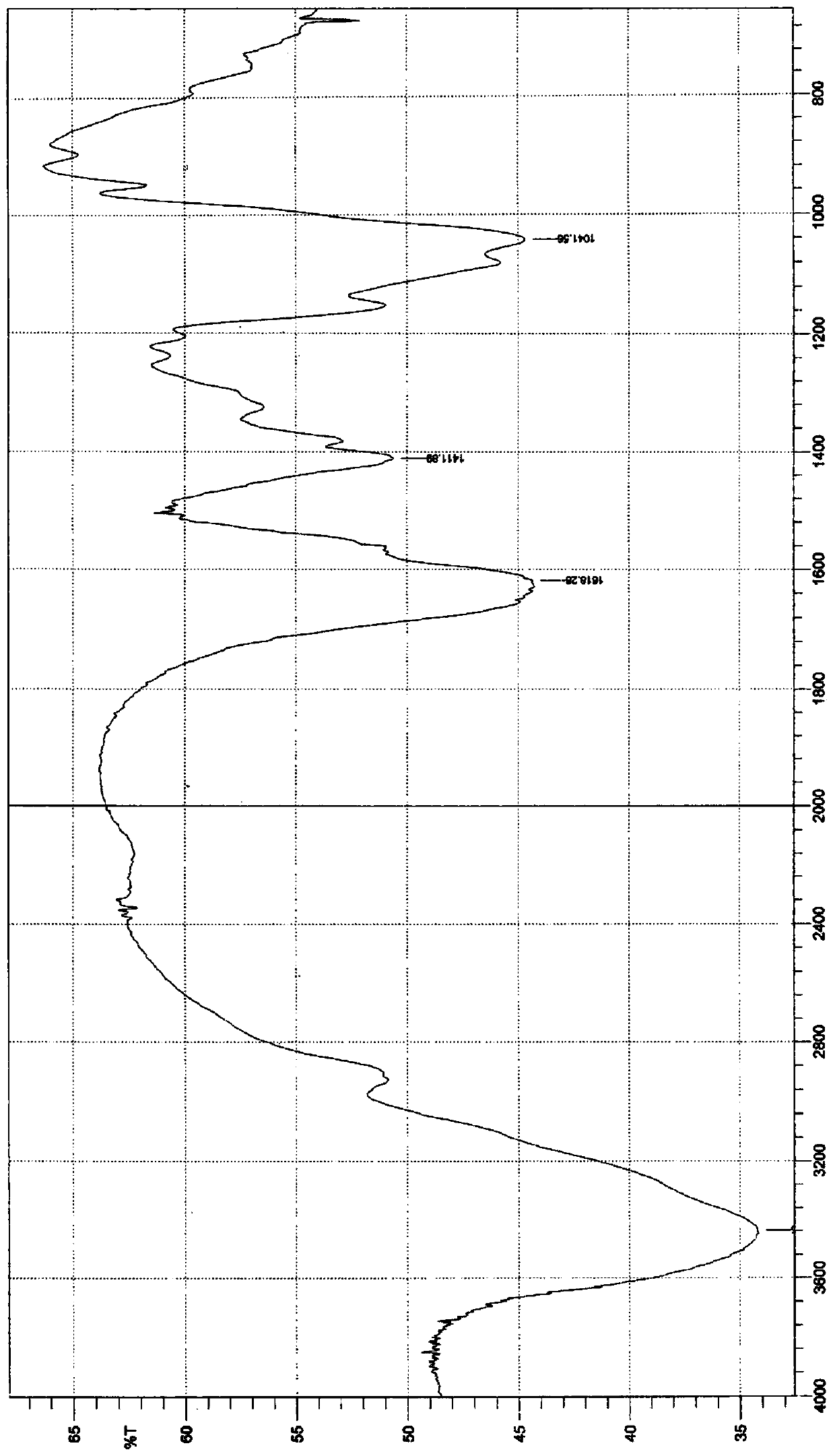
Image
Examples
Embodiment 1
[0038] Example 1 Synthesis of hyaluronic acid-chondroitin sulfate cross-linked product
[0039] (1) Dissolve 20g of sodium chloride and 5g of tetrabutylammonium bromide in 1000mL of distilled water for later use, weigh 20g of sodium hyaluronate with a molecular weight of 200kDa in a 2000mL reaction flask and dissolve it in the above distilled aqueous solution, then add 10g of EDC· After the HCl is completely dissolved, adjust the pH to 4.0 and react at room temperature for 3 hours, and check the pH every 15 minutes to keep it at 4.0;
[0040] (2) Weigh 20g of chondroitin sulfate and add it to the above reaction flask to dissolve, then add 10g of EDC·HCl to dissolve, adjust the pH to 4.0 and react at room temperature for 3h;
[0041] (3) Dilute the above solution to 10L for ultrafiltration, ultrafiltration until the solution is 1000mL, dilute the ultrafiltration again, and perform ultrafiltration three times to obtain 1000mL of effluent;
[0042] (4) Add 20g of sodium chloride...
Embodiment 2
[0043] Example 2 Synthesis of hyaluronic acid-heparan cross-linked product
[0044] (1) Dissolve 20g of sodium chloride and 5g of tetrabutylammonium bromide in 1000mL of distilled water for later use, weigh 20g of sodium hyaluronate with a molecular weight of 200kDa in a 2000mL reaction flask and dissolve it in the above distilled aqueous solution, then add 10g of EDC· After the HCl is completely dissolved, adjust the pH to 4.0 and react at room temperature for 3 hours, and check the pH every 15 minutes to keep it at 4.0;
[0045] (2) Weigh 20g of heparinoid and add it to the above reaction bottle to dissolve, then add 10g of EDC·HCl to dissolve, adjust the pH to 4.0 and react at room temperature for 3h;
[0046] (3) Dilute the above solution to 10L for ultrafiltration, ultrafiltration until the solution is 1000mL, dilute the ultrafiltration again, and perform ultrafiltration three times to obtain 1000mL of effluent;
[0047] (4) Add 20g of sodium chloride to the effluent to ...
Embodiment 3
[0048] Example 3 Detection of cross-linking degree of cross-linked chondroitin sulfate compounds
[0049] (1) Cross-linked chondroitin sulfate
[0050]Accurately weigh 5 g of cross-linked chondroitin sulfate prepared in Example 1, place in a 250 mL pressure-resistant bottle, transfer 20.00 mL of 0.5 mol / L sodium hydroxide standard solution with a pipette, and magnetically stir in a water bath at 50°C 30min to make it fully saponified, add 3 drops of phenolphthalein indicator, titrate with 0.25mol / L hydrochloric acid standard solution until colorless as the end point, measure in parallel three times, the volume of hydrochloric acid consumed is V 1 .
[0051] Follow the above steps, but do not add cross-linked chondroitin sulfate / sodium hyaluronate, conduct 3 blank experiments, and consume a volume of hydrochloric acid of V 2 .
[0052] Cross-linking degree x= .
[0053] (2) Cross-linked heparan
[0054] Accurately weigh 5 g of the cross-linked heparin prepared in Example...
PUM
| Property | Measurement | Unit |
|---|---|---|
| cross-linking degree | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com