A kind of preparation method of 8-amide-5-haloquinoline derivatives
A technology of haloquinoline and derivatives, which is applied in the field of preparation of 8-amide-5-haloquinoline derivatives, can solve the problems of not meeting the environmental protection requirements of green chemistry and poor environmental friendliness, and achieve easy amplification and promotion The effect of high growth and yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
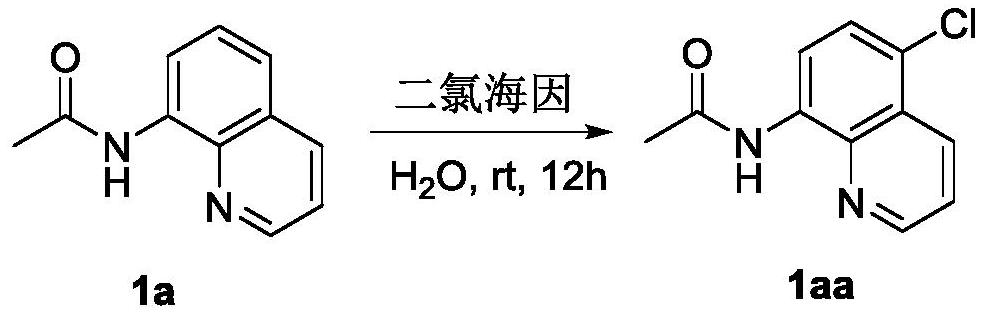
[0021] Adopt the preparation method of a kind of 8-amide-5-haloquinoline derivative of the present invention, prepare N-(5-chloroquinolin-8-base) acetamide (1aa), English name is N-(5-chloroquinolin- 8-yl)acetamide (1aa), its chemical reaction formula is as follows:
[0022]
[0023] The specific operation steps are: put 8-acetylaminoquinoline (186mg, 1mmol), dichlorohydantoin (140mg, 0.7mmol), benzyltriethylammonium chloride (TEBA) (45.6mg, 0.2mmol) into a 25ml reaction bottle mmol) and water 10mL, stirred and reacted at room temperature for 12h; the reaction solution was filtered and concentrated under reduced pressure to obtain a crude product, which was separated by column chromatography (eluent: petroleum ether: dichloromethane = 3:1~1:1) , to obtain 213.3 mg of white powdery solid (target compound I, 1aa), with a yield of 96.7% (calculated as 8-acetylaminoquinoline).
[0024] The NMR data of target compound I are:
[0025] 1 H NMR (300MHz, CDCl 3 )δ (ppm): 9.75 (s...
Embodiment 2
[0027] Adopt a kind of preparation method of 8-amide-5-haloquinoline derivatives of the present invention, prepare N-(5-bromoquinolin-8-base) acetamide (1ab), English name is N-(5-bromoquinolin -8-yl)acetamide (1ab), its chemical reaction formula is as follows:
[0028]
[0029] The specific operation steps are: put 8-acetamidoquinoline (93mg, 0.5mmol), dibromohydantoin (74.4mg, 0.26mmol), TEBA (22.8mg, 0.1mmol) and 10mL of water into a 25ml reaction bottle, and stir at room temperature Reacted for 12 hours; the reaction solution was filtered and concentrated under reduced pressure to obtain a crude product, which was separated by column chromatography (eluent: petroleum ether: dichloromethane = 3:1~1:1) to obtain a white powdery solid (target compound Ⅰ, 1ab) 123.7 mg, the yield was 93.4% (based on 8-acetylaminoquinoline).
[0030] The NMR data of target compound I are:
[0031] 1 H NMR (300MHz, DMSO-d 6 )δ (ppm): 10.20 (s, 1H), 9.00 (dd, J = 4.2, 1.4Hz, 1H), 8.57 (d, ...
Embodiment 3
[0033] Adopt a kind of preparation method of 8-amide-5-haloquinoline derivative of the present invention, prepare N-(5-iodoquinolin-8-base) acetamide (1ac), English name is N-(5-iodoquinolin -8-yl)acetamide (1ac), its chemical reaction formula is as follows:
[0034]
[0035] The specific operation steps are: put 8-acetamidoquinoline (93mg, 0.5mmol), diiodhydantoin (140mg, 0.35mmol), TEBA (22.8mg, 0.1mmol) and 10mL of water into a 20mL reaction bottle, and stir the reaction at room temperature 12h; the reaction solution was filtered and concentrated under reduced pressure to obtain a crude product, which was separated by column chromatography (eluent: petroleum ether:dichloromethane=3:1~1:1) to obtain a light brown solid (target compound I, 1ac) 142.0 mg, yield 91.0% (calculated as 8-acetylaminoquinoline).
[0036] The NMR data of target compound I are:
[0037] 1 H NMR (300MHz, DMSO-d 6 )δ (ppm): 10.22 (s, 1H), 9.02 (d, J = 2.6Hz, 1H), 8.56 (dd, J = 12.4, 8.5Hz, 2H), 7...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com