Application of sterol and pharmaceutically acceptable salts thereof in preparation of medicine for resisting hepatitis B virus
A hepatitis B virus and sterol technology, applied in the direction of antiviral agents, drug combinations, steroids, etc., can solve the problems of long course of treatment, easy accumulation of drug resistance, and large side effects, and achieve good anti-hepatitis B virus. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Extraction and separation of embodiment 1 sterol
[0024] 1. Preparation of snail extract:
[0025] Take 25kg of fresh sea snail samples, wash and remove the sediment, heat in boiling water for 3-5 minutes, remove the shell, freeze-dry the remaining soft tissue, grind the powder with a high-speed pulverizer, and put the powder in a 1000mL round bottom flask in batches, Add appropriate amount of 95% ethanol to reflux for extraction. Four extractions were performed, with each extraction taking 1 to 2 hours. Concentrate all the extracts by rotary evaporation, place them in a freeze dryer overnight after concentration, and obtain 95% ethanol extract, weighing 381 g.
[0026] The above-mentioned 95% ethanol extract is sequentially extracted with petroleum ether, dichloromethane, ethyl acetate and n-butanol, the extracted solution is combined and concentrated, that is, the sample is roughly divided into five phases and weighed, wherein the petroleum ether extract (APE) is ...
Embodiment 2
[0031] Embodiment 2 sterol in vitro activity screening
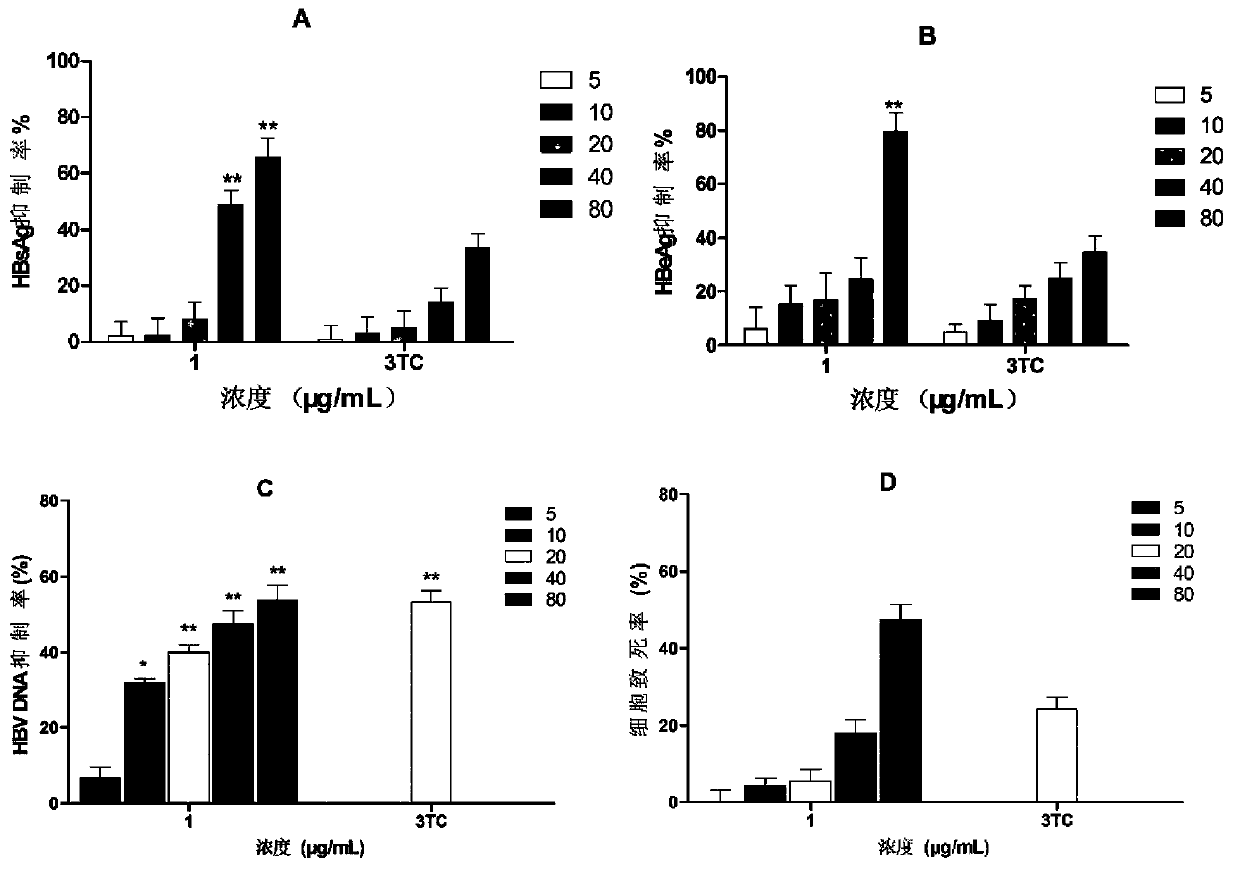
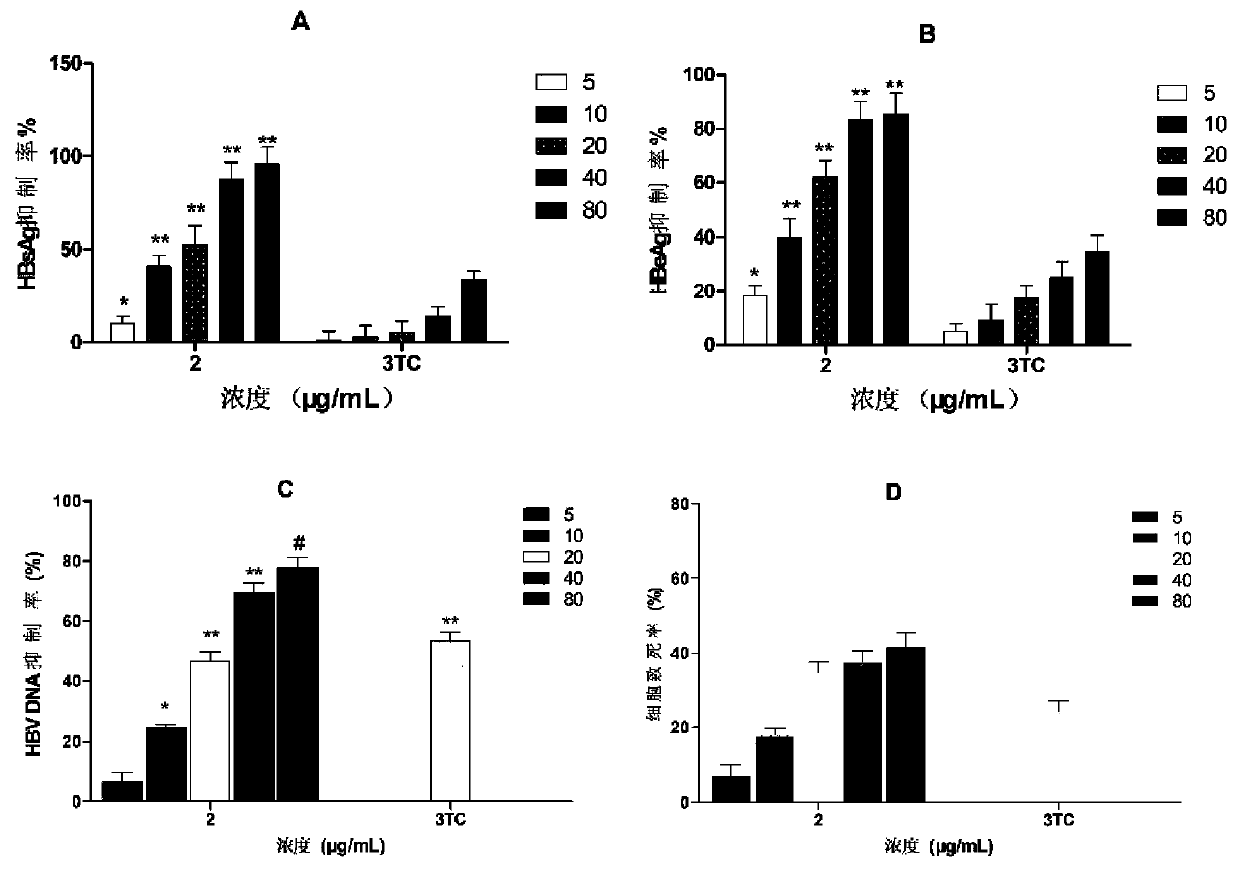
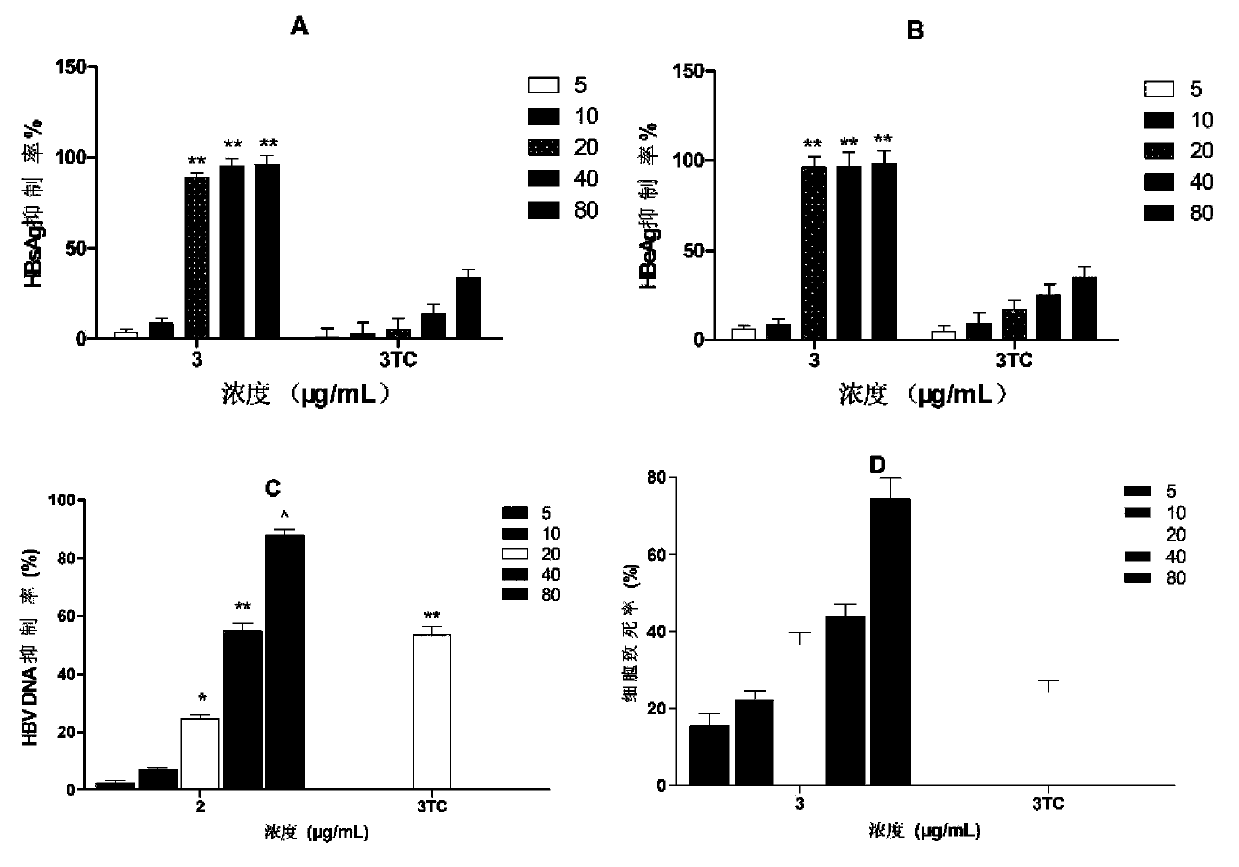
[0032] Hepatitis B virus antigen (HBsAg) and hepatitis B virus e antigen (HBeAg) are clinically important indicators for judging HBV infection. Hepatitis B surface antigen (HBsAg) is the coat protein of HBV. It is antigenic and not infectious, but its appearance is often accompanied by the presence of HBV, so it is a sign of HBV infection. HBeAg is the core internal component of the virus, and it is considered as a sign of virus replication in virus serological detection, indicating that there is progressive damage and high infectivity in liver cells. HBV DNA is HBV deoxyribonucleic acid, which is also the core part of the virus. It is the gold indicator for judging the replication of the virus. The quantitative examination of HBV DNA is the most direct indicator for detecting HBV infection. Clinically, quantitative detection of HBV content in the blood of hepatitis B patients can determine the infection status of patie...
Embodiment approach
[0037] a. Compound solution preparation: Dissolve 8.0 mg of compounds 1 to 3 in 500 μL DMSO, and make 4 gradient double dilutions in DMSO.
[0038] b. Cell plate: Dilute the HepG2.2.15 cells in the cell bottle to a concentration of 1×10 5 / mL, add 1mL cell suspension to each well of a 24-well plate, in CO 2 Place in the cell culture incubator for 24 hours, the culture conditions are 37°C, 5.0% CO 2 concentration.
[0039] c. Dosing: Add 5 μL of the drug solution prepared in the above 1 to each well of 1 to 5 of each row of the 24-well plate, so that the concentration of each compound in each well reaches 80 μg / mL, 40 μg / mL, 20 μg / mL, and 10 μg / mL. mL, 5 μg / mL, add 5 μL DMSO to the sixth well. The final concentration of DMSO in each well was 0.5%. in CO 2 Cell culture incubator, culture conditions 37 ℃, 5.0% CO 2 concentration. Aspirate the supernatant every three days, and re-add the complete medium and drug solution.
[0040] d. Add part of the above cell supernatant ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com