Fluorescent probe for rapidly identifying 2, 4, 6-trinitrophenol and preparation method thereof
A trinitrophenol, fluorescent probe technology, applied in fluorescence/phosphorescence, chemical instruments and methods, luminescent materials, etc., can solve the problems that water solubility, selectivity and visibility cannot be well balanced, and achieve The effect of low cost, good stability and simple synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
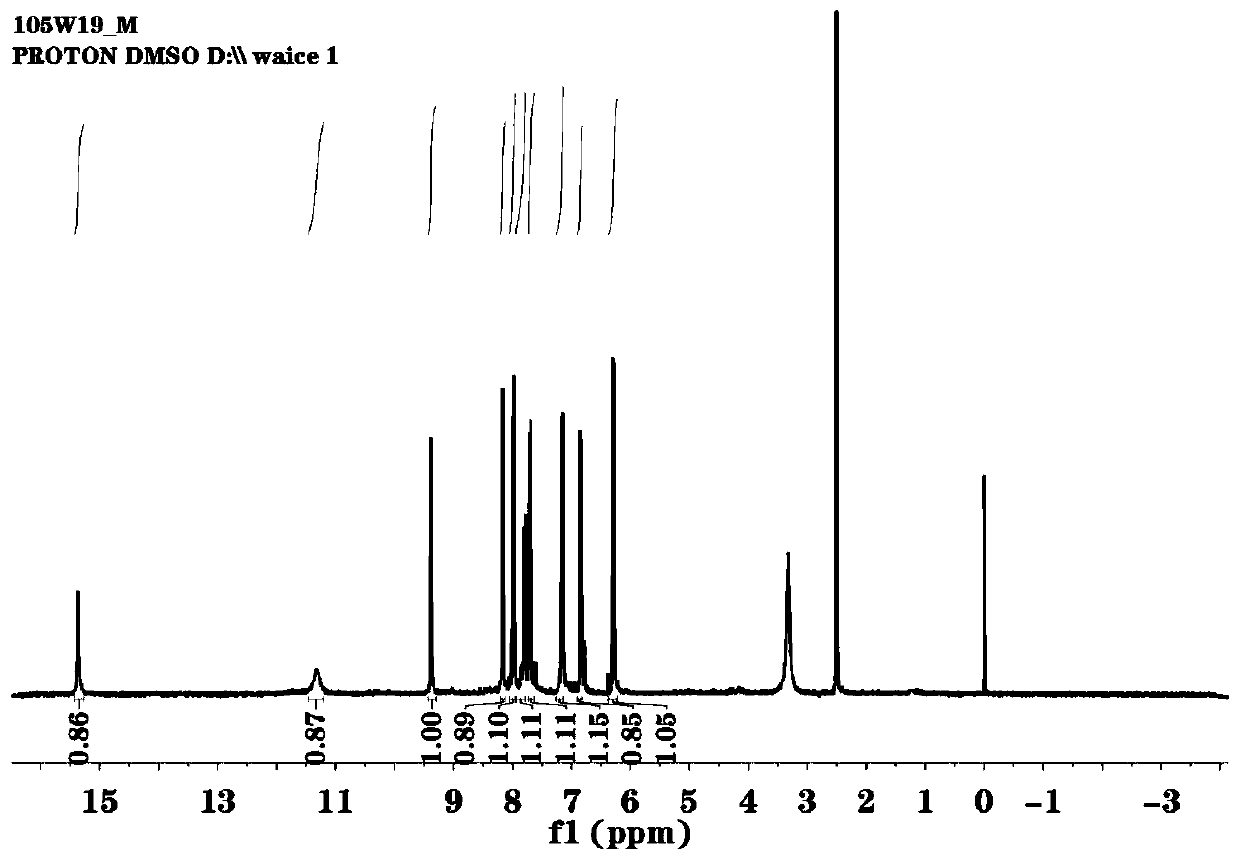
[0030] (1) Dissolve 2g (12.3mmol) of 7-hydroxycoumarin and 4g (28.5mmol) of hexamethylenetetramine in 35mL of glacial acetic acid, keep at 90°C for 9h under magnetic stirring, then add aqueous hydrochloric acid (V 盐酸 :V 水 =21:25) 46mL, and then heated to 70°C to continue the incubation reaction for 1h. Cooled to room temperature, poured into 150mL ice water, extracted with ethyl acetate (3 times, 50mL each time), combined the extracts with anhydrous Na 2 SO 4 After drying, the solvent ethyl acetate was removed by rotary evaporation to obtain the crude product of 8-formyl-7-hydroxycoumarin in the form of light yellow powder, which was recrystallized from absolute ethanol with a yield of 12%.
[0031] (2) Take 0.2g (1.05mmol) of the 8-formyl-7-hydroxycoumarin obtained in the above-mentioned embodiment 1 (1) and dissolve it in 35mL of absolute ethanol, stir and dissolve it, and then add it in 5mL of absolute ethanol dropwise. 0.1458 g (0.52 mmol) 3,3'-diamino-4,4'-dihydroxydip...
Embodiment 2
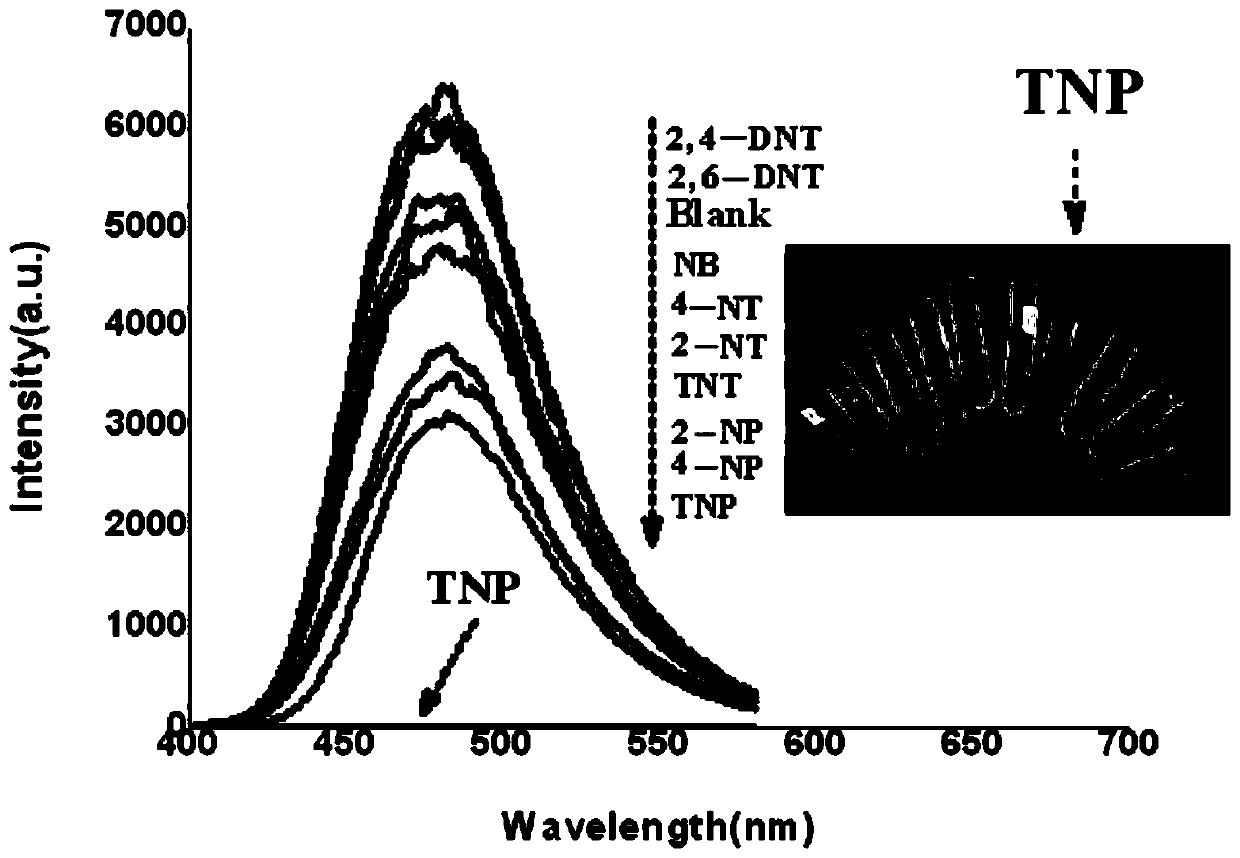
[0034] Embodiment 2: Measurement of the fluorescence spectrum response of conventional nitroaromatic explosives to probe L solution
[0035] The fluorescence spectral response of the probe to conventional nitroaromatic explosives was measured in DMF-H 2 O (V DMF / V H2O =9:1) carried out in the mixed solvent, the probe used is the probe sample prepared in embodiment 1, and the concentration of the probe and different conventional nitroaromatic explosives is 10 -3 mol / L.
[0036] With DMF-H 2 O(V DMF / V H2O =9:1) The mixed solution is prepared as a solvent with a concentration of 10 -3 mol / L of 4-nitrotoluene (4-NT), 2,6-dinitrotoluene (2,6-DNT), trinitrotoluene (TNT), 2,4-dinitrotoluene (2, 4-DNT), p-nitrophenol (4-NP), o-nitrophenol (2-NP), nitrobenzene (NB), 2-nitrotoluene (2-NT), 2,4,6-tri Nitrophenol (TNP) and fluorescent probe L solutions are used in the following examples.
[0037] Use a pipette gun to draw 1mL concentration of 10 -3 The mol / L fluorescent probe ...
Embodiment 3
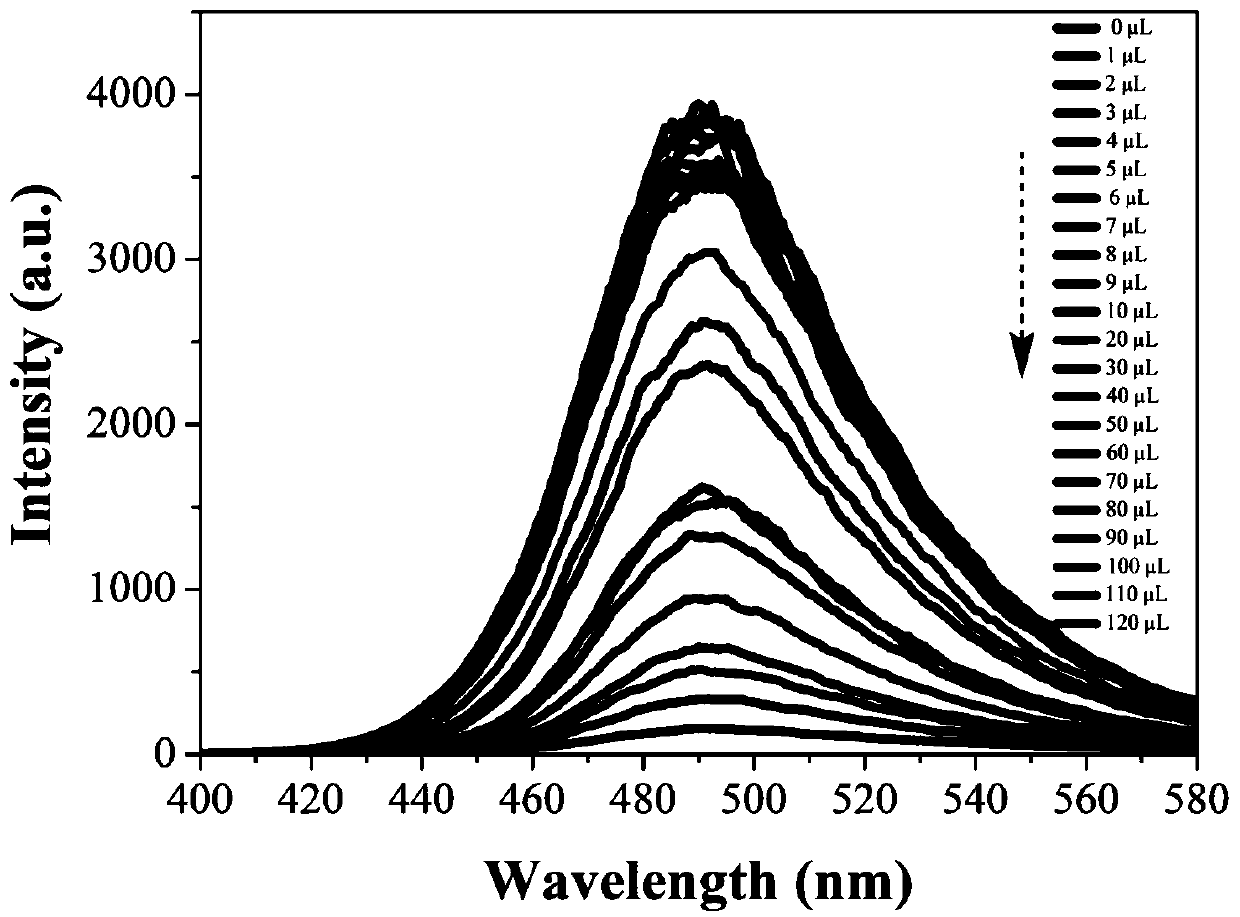
[0038] Embodiment 3: TNP is to the fluorescence titration of fluorescent probe L solution
[0039] Use a pipette to draw 2mL with a concentration of 10 -3 The fluorescent probe L solution of mol / L was transferred to a 3mL cuvette, and the dropping concentration was 10 -3 mol / L TNP solution, test the influence of TNP concentration on the fluorescent performance of the probe solution (λex=400nm, λem=580nm). The result is as image 3 shown. Depend on image 3 It can be seen that with the gradual addition of TNP, the intensity of the fluorescence emission peak of the system gradually decreases. The reason is that the three electron-deficient nitro groups in the TNP structure receive photon excitation and fall back to the electrons in the ground state, and the electrons cannot return to the original excited state. Fluorescence quenching results in fluorescence spectra of different intensities.
[0040] All the experimental conditions and processing methods of this embodiment a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com