A kind of atorvastatin calcium tablet and its preparation process
A technology of atorvastatin calcium and lubricant, which is applied in the field of atorvastatin calcium tablet and its preparation, can solve the problems of surfactant gastrointestinal irritation, failure to dissolve rapidly, and degradation of atorvastatin calcium. , to achieve the effect of improving disintegration rate and dissolution rate, improving various properties, and improving dissolution rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
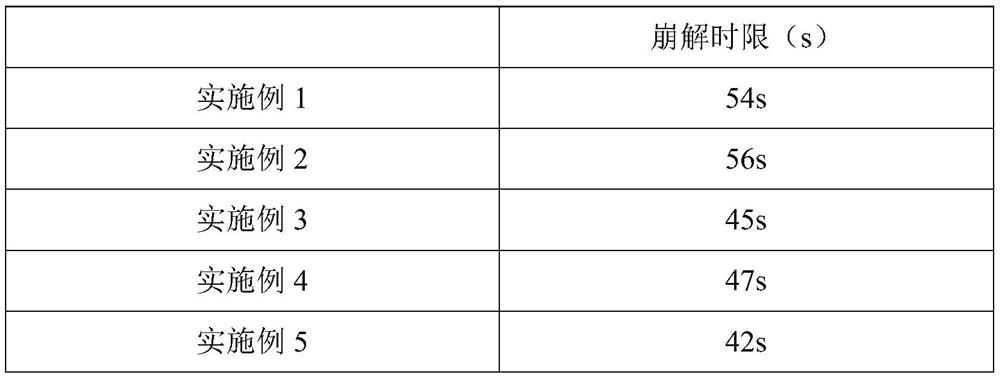
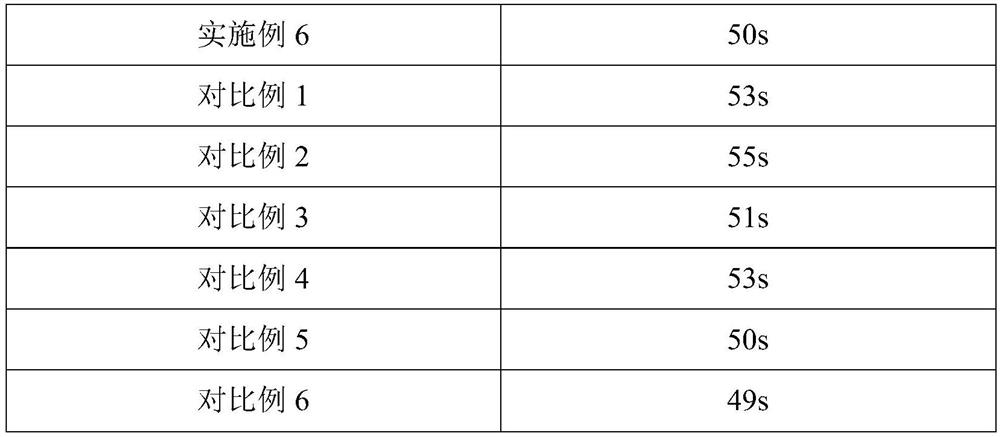
Embodiment 1
[0069] An atorvastatin calcium tablet, comprising the following components in parts by weight: 5 parts of atorvastatin calcium with a particle diameter of 30-40 μm, 40 parts of starch, 1 part of povidone, and 20 parts of crospovidone , 1 part of magnesium stearate, 3 parts of polyethylene glycol-4000.
[0070] Atorvastatin calcium tablets are prepared by the following method:
[0071] (1) Mix atorvastatin calcium, starch, 10% magnesium stearate, and crospovidone, first stir at 50r / min for 5min, and then 200r / min for 10min to obtain substance A;
[0072] (2) Heating purified water to 40°C, adding povidone and polyethylene glycol-4000 according to a mass ratio of 1:3, to obtain substance B;
[0073] (3) Mixing substance A and substance B, passing through a 16-mesh sieve to obtain wet granules;
[0074] (4) Dry the wet granules at 40°C, control the moisture to <2%, pass through a 20-mesh sieve for granulation, and obtain dry granules;
[0075] (5) Add the remaining 90% magnesi...
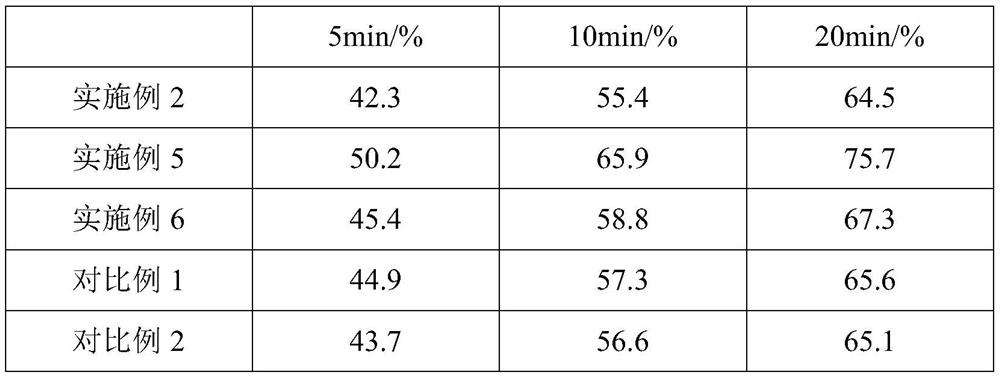
Embodiment 2
[0077] An atorvastatin calcium tablet, comprising the following components in parts by weight: 15 parts of atorvastatin calcium with a particle diameter of 30-40 μm, 70 parts of mannitol, 5 parts of hydroxypropyl cellulose, and carboxymethyl starch 35 parts of sodium, 5 parts of sodium stearate fumarate, 8 parts of polyethylene glycol-6000.
[0078] Atorvastatin calcium tablets are prepared by the following method:
[0079] (1) Mix atorvastatin calcium, mannitol, 20% sodium stearate fumarate, and sodium carboxymethyl starch, first stir at 100r / min for 10min, and then stir at 300r / min for 20min to obtain substance A;
[0080] (2) Heating purified water to 50°C, adding hydroxypropyl cellulose and polyethylene glycol-6000 according to the mass ratio of 1:10, to obtain substance B;
[0081] (3) mix substance A and substance B, pass through a 18-mesh sieve, and obtain wet granules;
[0082] (4) Dry the wet granules at 60°C, control the moisture to <2%, pass through a 20-mesh siev...
Embodiment 3
[0085] An atorvastatin calcium tablet, comprising the following components in parts by weight: 5 parts of atorvastatin calcium, 40 parts of microcrystalline cellulose, 1 part of binder, 20 parts of disintegrant, 1 part of silicon dioxide , 3 parts of macrogol-4000, 1 part of aspartame.
[0086] The particle size composition of the atorvastatin calcium is as follows: 30-40 μm: 5-10 μm=3:1;
[0087] The binder is a mixture of gelatin and hypromellose, and the mass ratio of the two is 1:1;
[0088] The disintegrant is a mixture of croscarmellose sodium, microcrystalline cellulose and low-substituted hydroxypropyl cellulose, and the mass ratio of the three is 1:3:2; agent, the croscarmellose sodium is an internal and external disintegrant (the mass ratio of the croscarmellose sodium and external disintegrant is 1:1), and the low-substituted hydroxypropyl cellulose Su is an internal disintegrant.
[0089] Atorvastatin calcium tablets are prepared by the following method:
[009...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com