Preparation method of high-energy-density and high-voltage graphite-zinc-based ion battery based on aqueous electrolyte
A high energy density, water-based electrolyte technology, applied in the field of preparation of water-based electrolyte graphite-zinc-based ion batteries, can solve the problems of narrow voltage window, low voltage, low energy density, etc., and achieve the effect of low price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] A method for preparing a high-energy density, high-voltage graphite-zinc-based ion battery based on an aqueous electrolyte, comprising the following steps:
[0030] (1) Synthesis of aqueous electrolyte:
[0031] The configuration concentration is 5.89mol kg -1 (30mol / L) zinc chloride and 0.38mol kg -1 (0.4mol / L) potassium bromide aqueous solution, the molar ratio of zinc chloride and potassium bromide in the aqueous solution is 750:1, as the electrolyte.
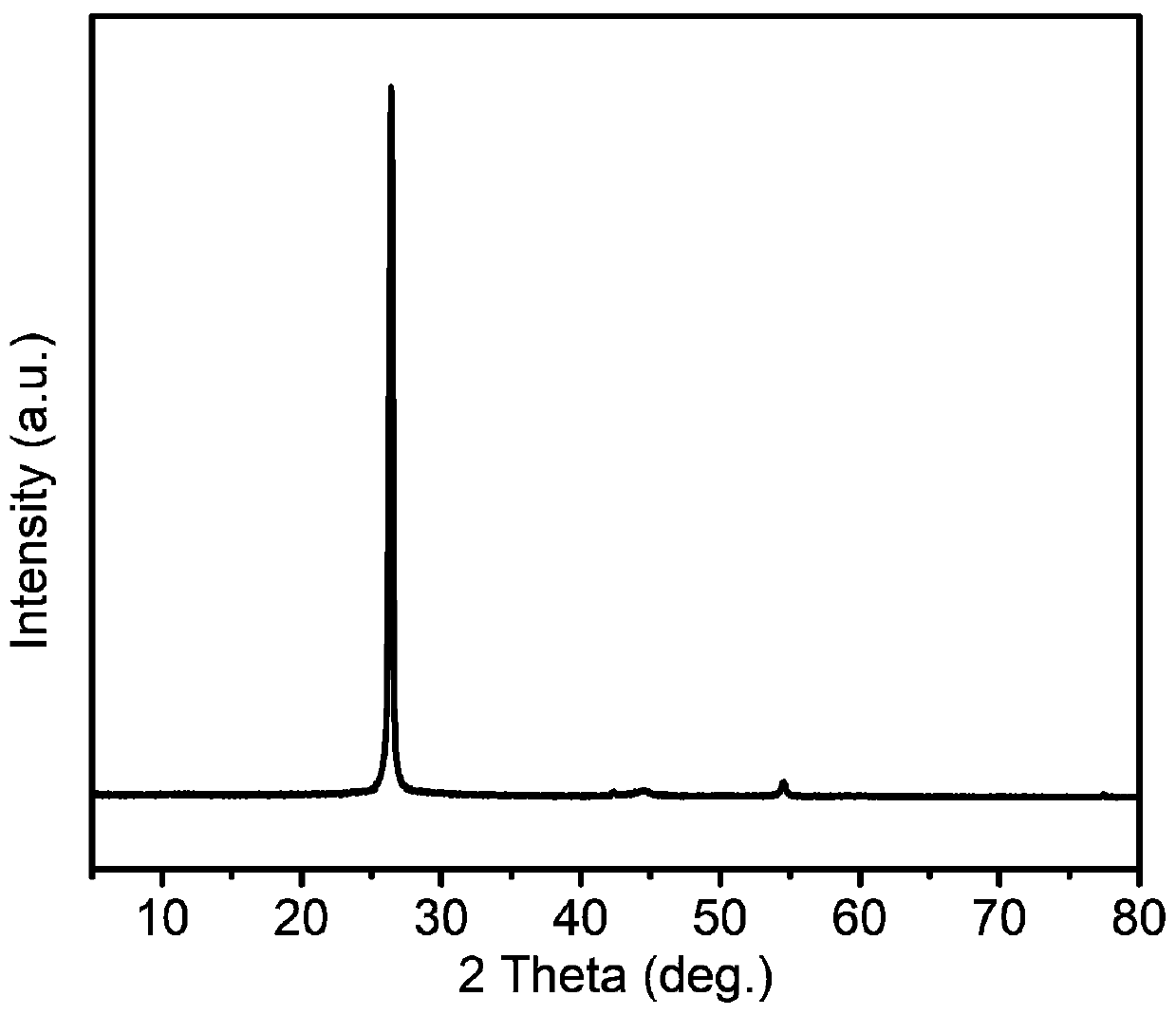
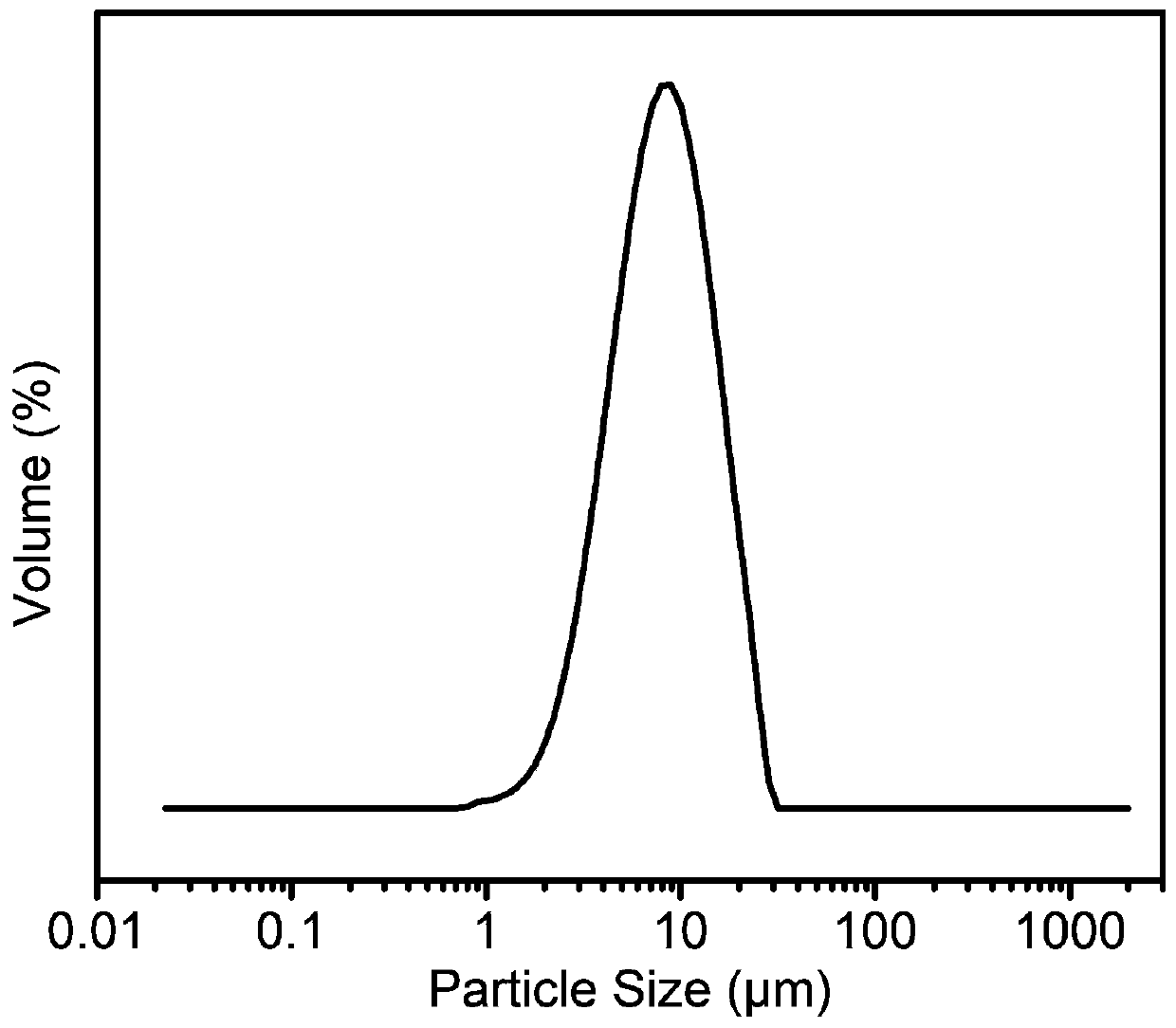
[0032] (2) 900 mg of artificial graphite positive electrode material and 3.33 g of 3% sodium carboxymethyl cellulose aqueous solution were fully stirred, then coated with a wet film-making method, and vacuum-dried at 80° C. for 10 hours to obtain a positive electrode sheet. The material characterization of the graphite cathode material used is as follows figure 1 , powder X-ray results show the phase purity of graphite; figure 2 The field emission scanning electron microscope image shows that the morphology of g...
Embodiment 2
[0035] A method for preparing a high-energy density, high-voltage graphite-zinc-based ion battery based on an aqueous electrolyte, comprising the following steps:
[0036] (1) Synthesis of electrolyte:
[0037] Dissolve zinc chloride (81.78g, 0.6mol) and potassium bromide (0.47g, 0.004mol) in water (20mL) to obtain potassium bromide (0.2mol / L) and zinc chloride (30mol / L) the electrolyte;
[0038] Dissolve zinc chloride (81.78g, 0.6mol) and potassium bromide (0.95g, 0.008mol) in water (20mL) to obtain potassium bromide (0.4mol / L) and zinc chloride (30mol / L) the electrolyte;
[0039]Dissolve zinc chloride (81.78g, 0.6mol) and potassium bromide (1.90g, 0.016mol) in water (20mL) to obtain potassium bromide (0.8mol / L) and zinc chloride (30mol / L) the electrolyte;
[0040] Dissolve zinc chloride (81.78g, 0.6mol) and potassium bromide (3.57g, 0.03mol) in water (20mL) to obtain potassium bromide (1.5mol / L) and zinc chloride (30mol / L) of electrolyte.
[0041] (2) 900 mg of artific...
Embodiment 3
[0044] A method for preparing a high-energy density, high-voltage graphite-zinc-based ion battery based on an aqueous electrolyte, comprising the following steps:
[0045] (1) Synthesis of electrolyte:
[0046] Dissolve zinc chloride (81.78g, 0.6mol) and potassium bromide (0.95g, 0.008mol) in water (20mL) to obtain potassium bromide (0.4mol / L) and zinc chloride (30mol / L) of electrolyte.
[0047] (2) 850 mg of artificial graphite positive electrode material, 50 mg of Super P conductive agent, and 3.33 g of 3% sodium carboxymethylcellulose aqueous solution were fully stirred, and then coated with a wet film-making method, and vacuum-dried at 60 ° C for 12 hours to obtain a positive electrode piece. The material characterization of the graphite cathode material used is as follows figure 1 , powder X-ray results show the high purity of graphite; figure 2 The field emission scanning electron microscope image shows that the morphology of graphite is micron-sized particles; i...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com