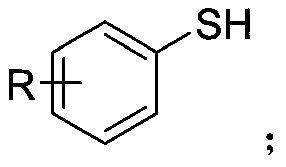
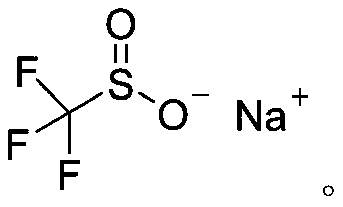
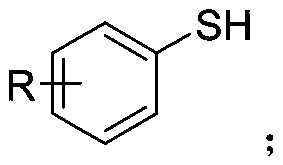
Synthetic method of aryl trifluoromethylthio compound
An aryl trifluoromethylthio group and a synthesis method technology are applied in the field of organic compound synthesis to achieve the effects of simple and safe operation, mild conditions and wide substrate applicability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029]
[0030] Add 4-methoxythiophenol (0.3mmol), sodium trifluoromethanesulfinate (0.9mmol), tetrabutylammonium tetrafluoroborate (0.3mmol) to a 25mL undivided electrolytic cell equipped with a magnetic stirrer. ), sodium bromide (0.6mmol), and then add 8.0mL acetonitrile; fix the reaction tube on a magnetic stirrer, add electrodes (graphite cathode, graphite anode) through 20mA constant current electrolysis, while uniformly stirring the reaction solution; the mixture is at room temperature After 8 hours of reaction at 25°C, the reaction is complete; add appropriate amount of water to the reaction solution, extract with ethyl acetate, dry with anhydrous sodium sulfate, and finally use a rotary evaporator to remove the solvent. The crude product is subjected to column chromatography (petroleum ether). : Ethyl acetate=30:1) The target product (3a) was obtained by separation and purification with a yield of 76%. The NMR data of this compound are: 1 HNMR(400MHz, CDCl 3 )δ7.57(d,J...
Embodiment 2
[0032]
[0033] Use 4-bromothiophenol (1b) instead of 4-methoxythiophenol (1a), and the others are the same as in Example 1. Column chromatography (petroleum ether) gave the target product (3b) with a yield of 60%. The NMR data of this compound are: 1 HNMR(600MHz, CDCl 3 )δ7.57-7.54(m,2H),7.52-7.49(m,2H). 13 C NMR(151MHz, CDCl 3 )δ137.81,132.87,131.46(q,J=309.55Hz),126.07,123.49(d,J=2.3Hz). 19 F NMR(565MHz, CDCl 3 )δ-42.64(s,3F).
Embodiment 3
[0035]
[0036] Use 4-acetylthiophenol (1c) instead of 4-methoxythiophenol (1a), and the others are the same as in Example 1. Column chromatography (dichloromethane) gave the target product (3c) with a yield of 66%. The NMR data of this compound are: 1 H NMR(600MHz, DMSO-d 6 )δ10.22(s,1H), 7.70--7.67(m,2H), 7.61--7.58(m,2H), 2.03(s,3H). 13 C NMR(151MHz, DMSO-d 6 )δ169.46,142.82,137.80,130.14(q,J=308.1Hz), 120.32,116.05(d,J=2.3Hz), 24.63. 19 FNMR(565MHz,DMSO-d 6 )δ-42.79(s, 3F).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com