Compositions and processes for targeted delivery, expression and modulation of coding ribonucleic acids in tissue
A composition and delivery technology, applied in the field of messenger ribonucleic acid delivery
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example
[0181] general agreement
[0182] cell line
[0183] Human liver cancer (HCC) HepG2 ( HB-8065 TM ) and Hep3B ( HB-8064 TM ) cells were purchased from ATCC. Cells were cultured in Eagle's Minimal Essential Medium (EMEM) (Cellgro, USA), 10% FBS (HyClone, USA), streptomycin (100 μg / mL) and penicillin (100 U / mL -1 ) (Cellgro) at 37°C in 5% CO 2 Cultivated in a single-layer form in an atmosphere. HepG2 cells were treated at 5 μg / cm 2 The collagen concentration was grown on collagen (Gibco, USA) coated plates.
[0184] HMCPP5 (pooled cultured human hepatocytes; a mixture of cultured primary hepatocytes generated by pooling cells from 5 individual donors) was purchased from ThermoFisher Scientific, USA. The cells were inoculated in Williams' E medium ( Williams E Medium, WEM). 24 hours after inoculation, the WEM / Mix A medium was changed to supplemented with 0.1 μM dexamethasone and Mix B (penicillin / streptomycin, ITS (human recombinant insulin, human transferrin, selen...
example 1
[0221] Example 1: Tumor-specific gene expression regulated by miRNA-122
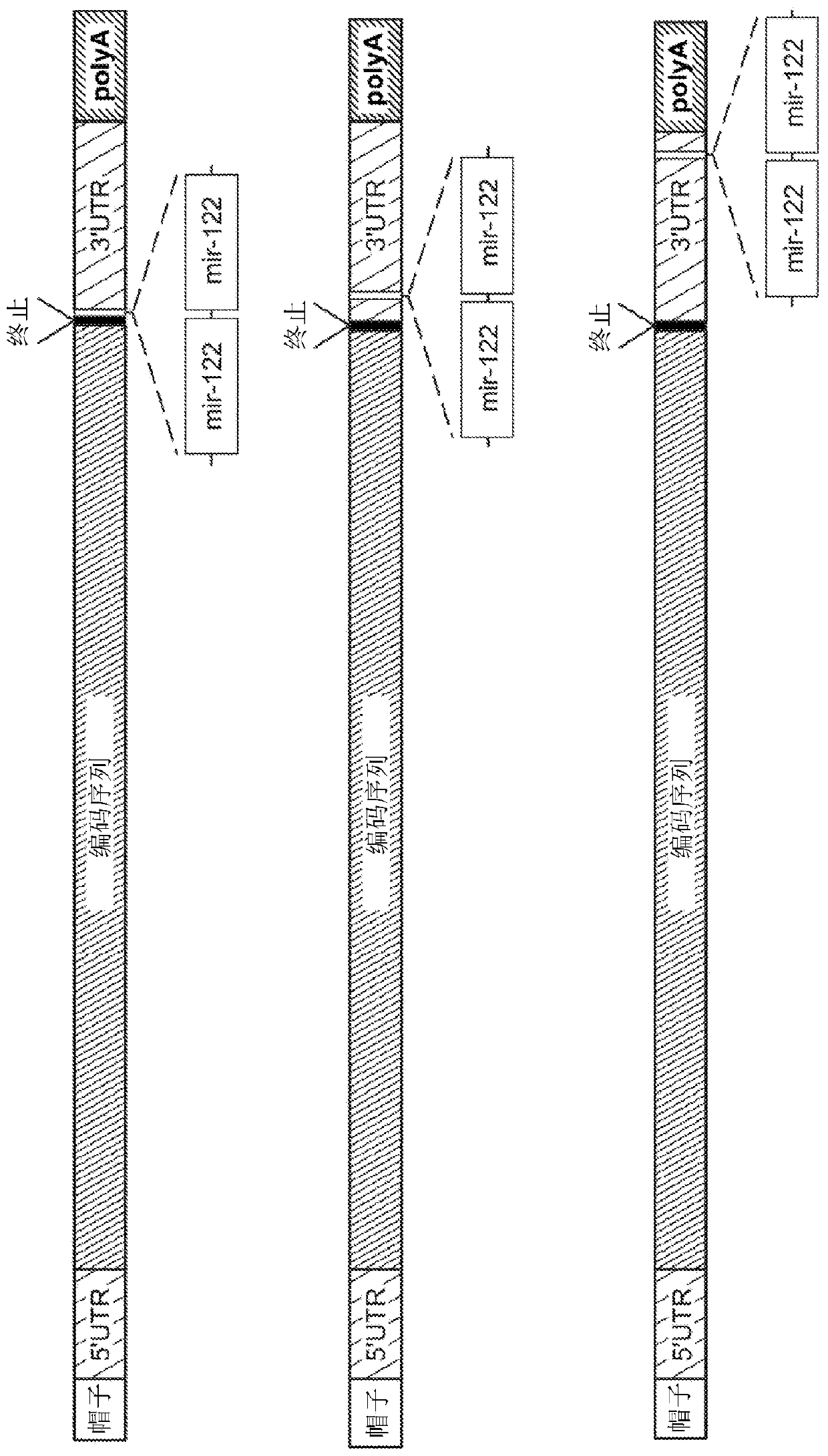
[0222] miRNA-122 is an abundant liver-specific miRNA whose expression is markedly reduced in human primary liver cancer (HCC) and HCC-derived cell lines such as Hep3B and HepG2. The purpose of this study was to demonstrate that by inserting the miRNA-122 target sequence (e.g., SEQ ID NO:2, as in image 3 modification of the 3' untranslated region (UTR) of the mRNA sequence described in variant 1) may allow translational repression and / or deadenylation followed by uncapping in normal hepatocytes but was not tested in Exogenous mRNA in HCC cell lines.
[0223] To examine endogenous miRNA-122 activity in healthy hepatocytes, using mCherry (red fluorescent protein) as the introduced gene of interest and following mCherry (red fluorescent protein) expression over time, mRNA-mCherry prepared according to the general protocol above or mRNA-mCherry-122 transfection of HMCPP5 cells (pooled cultured human hepato...
example 2
[0233] Example 2: Protein expression levels following tumor-specific gene expression
[0234] In another experiment, Western blot was used to determine the final protein expression level after transfection, as shown below.
[0235] Transfection and immunoblotting of cell lines - protein A
[0236] To assess tumor-specific expression levels of an exemplary 25kDa human protein (labeled 'Protein A'), hepatoma cells (HepG2 and Hep3B) and healthy hepatocytes (HMCPP5) were seeded into 12-well plates and treated with 0.5 μg / well Nanoformulated mRNA expressing human protein A, 25kDa (mRNA-A-DMP CTx ) or an mRNA expressing human protein A (a human protein of approximately 25 kDa) containing two miRNA122-binding sequences in the 3'UTR (SEQ ID NO:2), variant 1 (mRNA-A-miRNA122-DMP CTx ) transfection as described in Example 1 for mCherry transfection. Twenty-four hours after transfection, immunoblotting was performed after total protein extraction.
[0237] For immunoblotting, mediu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com