Combined rapid detection method for various avian antibodies
A detection method and antibody technology are applied in the field of combined rapid detection of various poultry antibodies, which can solve the problems of heavy repetitive sample addition and poor accuracy of detection technicians, and achieve the effects of low cost, high accuracy and simple operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
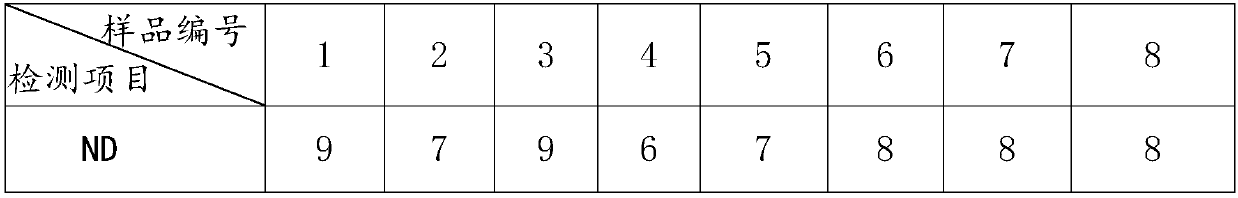
[0024] Step 1: Use an 8-channel micropipette to add 0.125 mL of physiological saline to the wells in the 1st to 10th columns of the 96-well original reaction plate 1.
[0025] Step 2: Use a single-channel micropipette to pipette 0.125mL of the serum solution to be tested to each well of the first column of the original reaction plate 1, and repeatedly pipette 5 times to mix.
[0026] Step 3: Use an 8-channel micropipette to draw 0.125mL from the wells of the first column of the original reaction plate 1, pipette and mix 5 times repeatedly, and then transfer to the wells of the second column. Pipette 0.125 mL into the wells and discard.
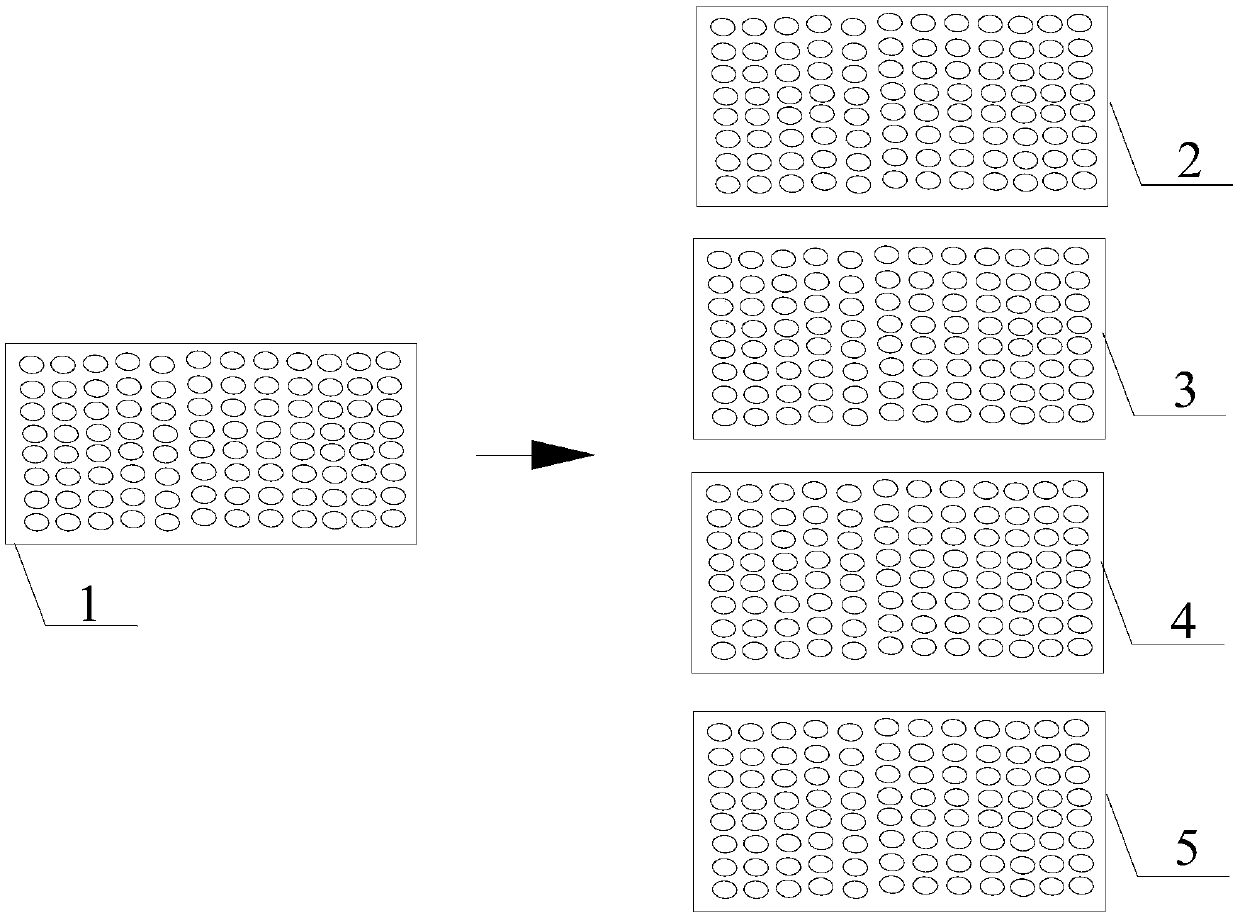
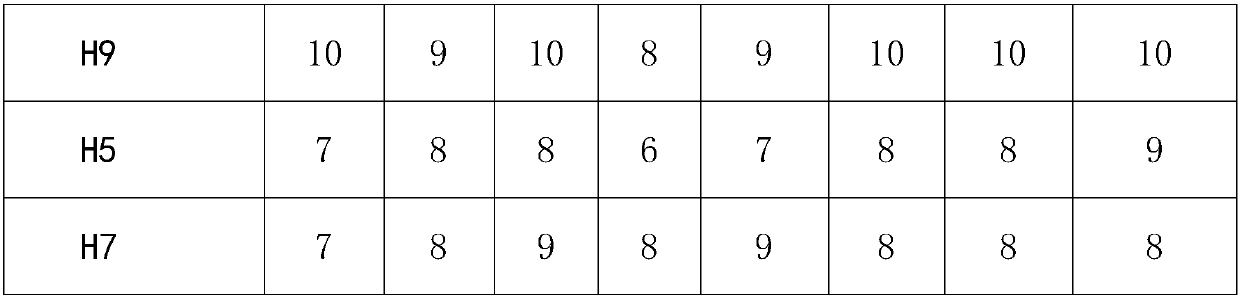
[0027] Step 4: If figure 1 As shown, transfer the serum to be tested diluted in step 3 into corresponding wells of 96-well ND reaction plate 2, H9 reaction plate 3, H5 reaction plate 4, and H7 reaction plate 5 at 0.025 mL per well.
[0028] Step 5: Add 0.025 mL of normal saline to the wells in column 11 of each reaction plate in step 4, and ...
Embodiment 2
[0037] Step 1: Use an 8-channel micropipette to add 0.125 mL of PBS to the 1st to 10th column wells of the 96-well original reaction plate 1.
[0038] Step 2: Use a single-channel micropipette to pipette 0.125mL of the serum solution to be tested to each well of the first column of the original reaction plate 1, and repeatedly pipette 5 times to mix.
[0039] Step 3: Use an 8-channel micropipette to draw 0.125mL from the wells of the first column of the original reaction plate 1, pipette and mix 5 times repeatedly, and then transfer to the wells of the second column. Pipette 0.125 mL into the wells and discard.
[0040] Step 4: If figure 1 As shown, transfer the serum to be tested diluted in step 3 into corresponding wells of 96-well ND reaction plate 2, H9 reaction plate 3, H5 reaction plate 4, and H7 reaction plate 5 at 0.025 mL per well.
[0041] Step 5: Add 0.025mL PBS to the wells in column 11 of each reaction plate in step 4, and add 0.05mL PBS to the wells in column 1...
Embodiment 3
[0045] Step 1: Use an 8-channel micropipette to add 0.1 mL of physiological saline to the wells in the 1st to 10th columns of the 96-well original reaction plate 1.
[0046] Step 2: Use a single-channel micropipette to pipette 0.1mL of the serum solution to be tested to each well of the first column of the original reaction plate 1, and repeatedly pipette 5 times to mix.
[0047] Step 3: Use an 8-channel micropipette to draw 0.1mL from each well of the first column of the original reaction plate 1 and pipette repeatedly 5 times to mix well, then transfer to the wells of the second column, and sequentially dilute to the wells of the 10th column. Pipette 0.1 mL into the wells and discard.
[0048] Step 4: If figure 1 As shown, transfer the serum to be tested diluted in step 3 into corresponding wells of 96-well ND reaction plate 2, H9 reaction plate 3, H5 reaction plate 4, and H7 reaction plate 5 at 0.02 mL per well.
[0049] Step 5: Add 0.02 mL of normal saline to the wells i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com